It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
reply to post by okiecowboy
A lot of Tokyo is contaminated beyond the levels found in the exclusion zones in Unkraine and Blearus. Tokyo is the most populated metropolitan area in the world. Populations around the Chrenobyl site were sparce with a few cities such as Chronobyl, or Pripiat. With popluations of 500,000 and 70,000 respectively at the time of the accident, these numbers are minute compared to the almost 36,000,000 people living in the Tokyo metropolitan area.
A lot of Tokyo is contaminated beyond the levels found in the exclusion zones in Unkraine and Blearus. Tokyo is the most populated metropolitan area in the world. Populations around the Chrenobyl site were sparce with a few cities such as Chronobyl, or Pripiat. With popluations of 500,000 and 70,000 respectively at the time of the accident, these numbers are minute compared to the almost 36,000,000 people living in the Tokyo metropolitan area.
I feel that I should note, that THIS:
www.abovetopsecret.com...
Was the simplified version of calculating your effective dose of radiation.
I left out the different decay paths of tritium (I only used the most common) and averaging out their energy level, and a more complete explanation of flux density and the inverse square law.
www.abovetopsecret.com...
Was the simplified version of calculating your effective dose of radiation.
I left out the different decay paths of tritium (I only used the most common) and averaging out their energy level, and a more complete explanation of flux density and the inverse square law.
Originally posted by ErtaiNaGia
I feel that I should note, that THIS:
www.abovetopsecret.com...
Was the simplified version of calculating your effective dose of radiation.
I left out the different decay paths of tritium (I only used the most common) and averaging out their energy level, and a more complete explanation of flux density and the inverse square law.
Yeah I was going to say for shorter less complicated explanation of your comversion see my post above your's, but I didn't want to detract from your great post and systematic explanation of does rates but I feel that it may be hard to folllow for people who don't have extended knowledge of the subject. Thanks for the contribution .
reply to post by BriGuyTM90
You are quite welcome... I am still trying to find a simpler way to explain it, so that more people can understand it easier...
Alas.... Geometry is a complicated mathematical subject, especially when you are dealing with Nuclear Physics.
Thanks for the contribution
You are quite welcome... I am still trying to find a simpler way to explain it, so that more people can understand it easier...
Alas.... Geometry is a complicated mathematical subject, especially when you are dealing with Nuclear Physics.
reply to post by ErtaiNaGia
In your explanation..your point of exposure is a 1 centimeter sphere correct?
In your explanation..your point of exposure is a 1 centimeter sphere correct?
reply to post by okiecowboy
In order to accurately calculate the exposure of radiation flux, it is necessary to determine flux falloff from distance by using the inverse square rule.
A good way to explain this, is to imagine a light bulb.
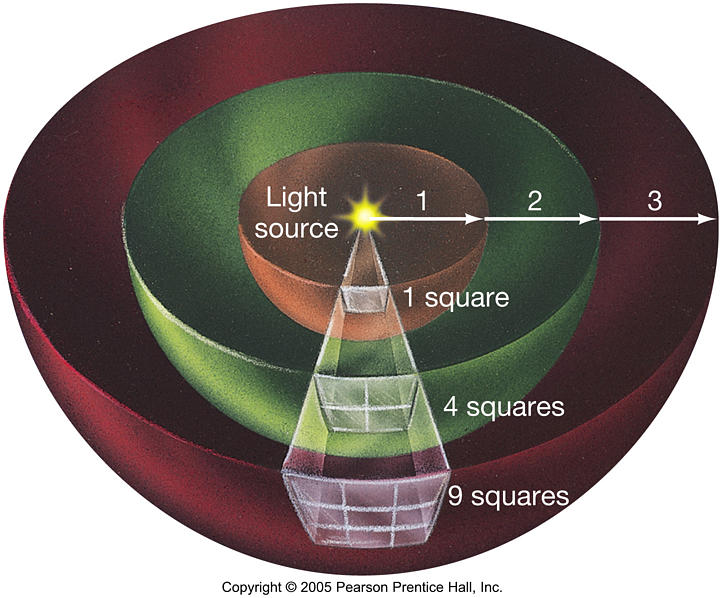
Let's say that this light-bulb with a surface area of one square centimetre, puts out 10 watts of light energy.
So, at the surface of the light bulb, the luminous flux intensity is 10 watts per square centimetre.
As the distance from the lightbulb increases, the 10 watts of energy is spread out over a sphere of larger and larger surface area, therefore the flux density per square centimetre decreases in proportion.
If you were to wrap your hand around the lightbulb, your hand would be absorbing all 10 watts of light energy.
If the lightbulb was right next to you, you would only be absorbing abour 50% of the total output energy, because only half the light is actually striking your body.
Necessarily, as you get farther away from the bulb, your body is absorbing less and less of the total output energy, because your angular size (as determined from the lightbulb) is decreasing in proportion to your distance.
In your explanation..your point of exposure is a 1 centimeter sphere correct?
In order to accurately calculate the exposure of radiation flux, it is necessary to determine flux falloff from distance by using the inverse square rule.
A good way to explain this, is to imagine a light bulb.

Let's say that this light-bulb with a surface area of one square centimetre, puts out 10 watts of light energy.
So, at the surface of the light bulb, the luminous flux intensity is 10 watts per square centimetre.
As the distance from the lightbulb increases, the 10 watts of energy is spread out over a sphere of larger and larger surface area, therefore the flux density per square centimetre decreases in proportion.
If you were to wrap your hand around the lightbulb, your hand would be absorbing all 10 watts of light energy.
If the lightbulb was right next to you, you would only be absorbing abour 50% of the total output energy, because only half the light is actually striking your body.
Necessarily, as you get farther away from the bulb, your body is absorbing less and less of the total output energy, because your angular size (as determined from the lightbulb) is decreasing in proportion to your distance.
edit on 26-4-2013 by ErtaiNaGia because: (no reason
given)
reply to post by BriGuyTM90
This Japanese real time map of radiation from Japan seems to have more of the darker colors hitting us on the West Coast US then it did before, if the incoming is constant should there not be a build up of particles?
www.zamg.ac.at...
This Japanese real time map of radiation from Japan seems to have more of the darker colors hitting us on the West Coast US then it did before, if the incoming is constant should there not be a build up of particles?
www.zamg.ac.at...
reply to post by ErtaiNaGia
But doesn't the radiation travel by particles and not radiation as put off by a light bulb? So if a big wind carries more you get more even if at a distance?
But doesn't the radiation travel by particles and not radiation as put off by a light bulb? So if a big wind carries more you get more even if at a distance?
reply to post by Char-Lee
There are Four different types of ionizing radiation.
Alpha Particle (Essentially high speed helium atoms)
Beta Particle (High Speed Electrons)
Neutron Radiation (Er.... High Speed Neutrons)
Gamma Radiation (Gamma and high energy X-Ray Photons)
So, Ionizing Radiation is BOTH particle, AND photons.
To answer your question, the speed and direction of the wind does not effect the "Range" of the emitted radiation, because the radiation causes damage primarily by it's great speed (Kinetic, or in the case of photons, it's energy)
In other words, if the Wind is even effecting the Particles at all, then the particle has already lost its initial energy TO the atoms in the air.
IT's sort of like asking if the ocean currents will make a bullet fired underwater travel any further....
This is because a Particle of radiation is so much smaller than an atom, and the Wind and Water moves things primarily because the atoms are impacting the object on one side with more force than the other sides.
Since the Radiation particle is so much smaller than the atoms, if the particle even TOUCHES a single atom, it loses all of its energy TO that atom.
But doesn't the radiation travel by particles and not radiation as put off by a light bulb? So if a big wind carries more you get more even if at a distance?
There are Four different types of ionizing radiation.
Alpha Particle (Essentially high speed helium atoms)
Beta Particle (High Speed Electrons)
Neutron Radiation (Er.... High Speed Neutrons)
Gamma Radiation (Gamma and high energy X-Ray Photons)
So, Ionizing Radiation is BOTH particle, AND photons.
To answer your question, the speed and direction of the wind does not effect the "Range" of the emitted radiation, because the radiation causes damage primarily by it's great speed (Kinetic, or in the case of photons, it's energy)
In other words, if the Wind is even effecting the Particles at all, then the particle has already lost its initial energy TO the atoms in the air.
IT's sort of like asking if the ocean currents will make a bullet fired underwater travel any further....
This is because a Particle of radiation is so much smaller than an atom, and the Wind and Water moves things primarily because the atoms are impacting the object on one side with more force than the other sides.
Since the Radiation particle is so much smaller than the atoms, if the particle even TOUCHES a single atom, it loses all of its energy TO that atom.
edit on 26-4-2013 by ErtaiNaGia because: (no reason given)
reply to post by Char-Lee
You confusing radioactive contaminants with the radiation given of by the radioactive isotopes. The contaminantss can spread by wind, water and a lot of diffrent ways. The particles given off by the radio isotopes (alpha, and beta)don't travel very far. Gama radiation can travel quite a distance but they are not effected by wind sense they are a high energy electromagnetic wave. So the worry is about spreading the radioactive materials and not the radiati on they give off.
You confusing radioactive contaminants with the radiation given of by the radioactive isotopes. The contaminantss can spread by wind, water and a lot of diffrent ways. The particles given off by the radio isotopes (alpha, and beta)don't travel very far. Gama radiation can travel quite a distance but they are not effected by wind sense they are a high energy electromagnetic wave. So the worry is about spreading the radioactive materials and not the radiati on they give off.
edit on 26-4-2013 by BriGuyTM90 because: (no reason given)
reply to post by BriGuyTM90
Radioisotope is the correct terminology to refer to Unstable Atoms that will eventually decay into other elements, and give off radiation.
Yes, Radioisotopes can be spread by any normal method that is capable of moving atoms.
However, wind and water transportation tends to dilute and disperse the concentrations of these radioisotopes, making them less dangerous in proportion to how much they are diluted.
To give you a good example (Rule of thumb) it is estimated that the Oceans naturally contain about 10,000,000,000,000 kilograms of Radioactive Uranium (10 trillion kilograms, or 250,000,000,000,000 Becquerels worth.), Diluted, and dispersed, of course.
en.wikipedia.org...
In addition to this, the Biosphere of the Earth contains roughly 11,000,000,000,000,000,000 Becquerels worth of Carbon-14
en.wikipedia.org...
You confusing radioactive contaminants with the radiation given of by the radioactive isotopes.
Radioisotope is the correct terminology to refer to Unstable Atoms that will eventually decay into other elements, and give off radiation.
The contaminantss can spread by wind, water and a lot of diffrent ways.
Yes, Radioisotopes can be spread by any normal method that is capable of moving atoms.
However, wind and water transportation tends to dilute and disperse the concentrations of these radioisotopes, making them less dangerous in proportion to how much they are diluted.
To give you a good example (Rule of thumb) it is estimated that the Oceans naturally contain about 10,000,000,000,000 kilograms of Radioactive Uranium (10 trillion kilograms, or 250,000,000,000,000 Becquerels worth.), Diluted, and dispersed, of course.
en.wikipedia.org...
In addition to this, the Biosphere of the Earth contains roughly 11,000,000,000,000,000,000 Becquerels worth of Carbon-14
en.wikipedia.org...
The report you cite is a little strange. A Becquerel is a measurement of nuclear disintegration in a second. So the unit is in terms of an inverse
second, and not in Bq/kg. Maybe they mean that one kg of the mud was measured to emit that amount of Bq Or someone may have confused a bec querel with
a Gray, which is measured in terms of kilograms. 1 Gray is the absorbtion of 1 joule of ionizing radiation per kilogram of mass.
Like any SI unit, Bq can be prefixed; commonly used multiples are kBq (kilobecquerel, 103 Bq), MBq (megabecquerel, 106 Bq), GBq (gigabecquerel, 109 Bq), TBq (terabecquerel, 1012 Bq), and PBq (petabecquerel, 1015 Bq). For practical application, 1 Bq is a small unit; therefore, the prefixes are common
I usually see kBq being used. So divide that by 1000 and you have 1000 Kilobecquerels, a less intimidating number.
reply to post by ErtaiNaGia
Thank you. I was wondering why do they have that website, what is its purpose?
Thank you. I was wondering why do they have that website, what is its purpose?
reply to post by Char-Lee
IT is an atmospheric model of prevailing wind currents that is used to estimate where, and in what concentrations airborne radioisotopes may travel to.
But you have to remember that the most important part is your DOSE.
The radioisotopes that are being released from the reactor, are dispersing, and diffusing over a *VERY* large volume of air.
And, if you remember correctly from my earlier posts, Once a particle of radiation is emitted, it doesn't travel that far... maybe a few inches to a few feet from the original site of decay.
If you are near a GREAT BIG QUANTITY of radioisotopes... you are probably going to have a bad day.
But if those same radioisotopes are spread out over MILLIONS AND BILLIONS of Cubic KILOMETRES..... then you are probably not going to be effected to any degree that could be measurable.
Thank you. I was wondering why do they have that website, what is its purpose?
IT is an atmospheric model of prevailing wind currents that is used to estimate where, and in what concentrations airborne radioisotopes may travel to.
But you have to remember that the most important part is your DOSE.
The radioisotopes that are being released from the reactor, are dispersing, and diffusing over a *VERY* large volume of air.
And, if you remember correctly from my earlier posts, Once a particle of radiation is emitted, it doesn't travel that far... maybe a few inches to a few feet from the original site of decay.
If you are near a GREAT BIG QUANTITY of radioisotopes... you are probably going to have a bad day.
But if those same radioisotopes are spread out over MILLIONS AND BILLIONS of Cubic KILOMETRES..... then you are probably not going to be effected to any degree that could be measurable.
reply to post by ErtaiNaGia
It's worse than that. 68 halvings isn't 1/4624. It's 2^-68.
Look at three halvings. 1/2 * 1/2 * 1/2 is 1/8, or 2^-3.
68 halvings is 2^-68, or about 3.4E-21.
Didn't check the rest of the math but the halving part makes the dose less than your estimate by about 18 orders of magnitude, so it goes from diddly to closely approximating zero.
But your statement about how you'd calculate the dose seems reasonable at first glance.
It's worse than that. 68 halvings isn't 1/4624. It's 2^-68.
Look at three halvings. 1/2 * 1/2 * 1/2 is 1/8, or 2^-3.
68 halvings is 2^-68, or about 3.4E-21.
Didn't check the rest of the math but the halving part makes the dose less than your estimate by about 18 orders of magnitude, so it goes from diddly to closely approximating zero.
But your statement about how you'd calculate the dose seems reasonable at first glance.
reply to post by Bedlam
Lol... that's funny... because I did 68^2
Yeah... but what is 18 orders of magnitude less than an atto-Sievert?
Thank you... I was more trying to explain exactly how Dosage is calculated, than to come up with a perfectly accurate answer...
I am bound to make some trifling mistakes such as the one you pointed out..... That was quite a bit of math.
If you happen to find any more errors, I would appreciate you letting me know, for future reference, of course.
isn't 1/4624. It's 2^-68.
Lol... that's funny... because I did 68^2
Didn't check the rest of the math but the halving part makes the dose less than your estimate by about 18 orders of magnitude
Yeah... but what is 18 orders of magnitude less than an atto-Sievert?
But your statement about how you'd calculate the dose seems reasonable at first glance.
Thank you... I was more trying to explain exactly how Dosage is calculated, than to come up with a perfectly accurate answer...
I am bound to make some trifling mistakes such as the one you pointed out..... That was quite a bit of math.
If you happen to find any more errors, I would appreciate you letting me know, for future reference, of course.
The sad thing is...the levels of cesium in these pools has doubled since this time last year....but they didn't want to drain the pools because of
affecting farms in the area...but let the kids go to school there
Originally posted by Char-Lee
reply to post by BriGuyTM90
This Japanese real time map of radiation from Japan seems to have more of the darker colors hitting us on the West Coast US then it did before, if the incoming is constant should there not be a build up of particles?
www.zamg.ac.at...
There should be a build up of contaminants if the deposition rate exceeds the rate of decay. So with an isotope like I131 with a relatively short have life of 8 days your going to have less of a build up then Sr90 with half life of 28.8 years if the rate of deposition is the same. This doesn't take into account bioaccumulation and dilution due to rain and other nature phenomena.
new topics
-
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 1 hours ago -
America's Greatest Ally
General Chit Chat: 2 hours ago -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 7 hours ago -
Maestro Benedetto
Literature: 8 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 8 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 7 hours ago, 27 flags -
Weinstein's conviction overturned
Mainstream News: 16 hours ago, 8 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 13 hours ago, 8 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 15 hours ago, 7 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 12 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 15 hours ago, 5 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 13 hours ago, 4 flags -
Is AI Better Than the Hollywood Elite?
Movies: 8 hours ago, 3 flags -
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 1 hours ago, 3 flags -
Maestro Benedetto
Literature: 8 hours ago, 1 flags
active topics
-
Russia Ukraine Update Thread - part 3
World War Three • 5732 • : F2d5thCavv2 -
The Acronym Game .. Pt.3
General Chit Chat • 7751 • : F2d5thCavv2 -
Salvador Dali's Moustaches
People • 28 • : zosimov -
Is AI Better Than the Hollywood Elite?
Movies • 17 • : ThePsycheaux -
The best Rice dish i've ever tasted... Kimchi Rice
Food and Cooking • 26 • : lamhaocc -
A Warning to America: 25 Ways the US is Being Destroyed
New World Order • 1 • : 727Sky -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest • 15 • : tarantulabite1 -
America's Greatest Ally
General Chit Chat • 1 • : BingoMcGoof -
How ageing is" immune deficiency"
Medical Issues & Conspiracies • 35 • : annonentity -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 49 • : Freeborn
