It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
a reply to: deadeyedick
No, I just showed clearly that 1000 W/min IS NOT equivalent to 60,000 J. It is equivalent to 60,000 J/s^2.
No, I just showed clearly that 1000 W/min IS NOT equivalent to 60,000 J. It is equivalent to 60,000 J/s^2.
a reply to: iterationzero
yea you gotta speak english to me
are you saying that 1000 min is a kWh?
i agree there has to be something i am missing here
even if i use the online conversions i get 60,000 joules
i honestly do not know what you are meaning with the symbols
you say j/s but the unit j already dictates a set time of 1 sec so there is no need to reference time again.
do you mean 60000 to the second power? cause that is incorrect i believe
yea you gotta speak english to me
are you saying that 1000 min is a kWh?
i agree there has to be something i am missing here
even if i use the online conversions i get 60,000 joules
i honestly do not know what you are meaning with the symbols
you say j/s but the unit j already dictates a set time of 1 sec so there is no need to reference time again.
do you mean 60000 to the second power? cause that is incorrect i believe
edit on 12-1-2015 by deadeyedick because: (no reason given)
I'm not sure what exactly you're trying to do but I can try to guess where your 82.7% came from:
originally posted by: DenyObfuscation
a reply to: Arbitrageur
3.658 kWh = 13.17 MJ
1 L of water - 112 g hydrogen = 15.91 MJ
If those figures are accurate a self powered 'loop' would require 82.7% efficiency to break even?
Could such a thing even be possible? I would think not.
Electrolysis
The other source I used said it's 237.1 kJ so I've been trying to use that figure consistently to be consistent, however I'm aware of the 286 kJ figure and what it represents, and it also is accurate as described above.
The electrolysis of water in standard conditions requires a theoretical minimum of 237 kJ of electrical energy input to dissociate each mole of water, which is the standard Gibbs free energy of formation of water. It also requires energy to overcome the change in entropy of the reaction. Therefore, the process cannot proceed below 286 kJ per mol if no external heat/energy is added.
If you divide 237 by 286 you get something close to your 82.7% figure so I'm guessing that's the origin, but it won't enable a self-powered loop. It means the 237 is a theoretical value that is the absolute best you could possibly do in terms of electrolyzer efficiency, but the most efficient electrolyzers are never that efficient. If that's not it maybe you could explain in more detail.
Joule doesn't dictate any time. A joule can be produced over a microsecond or a year or any other time interval.
originally posted by: deadeyedick
you say j/s but the unit j already dictates a set time of 1 sec so there is no need to reference time again.
edit on 12-1-2015 by Arbitrageur because: clarification
a reply to: deadeyedick
I am.
No. I have no idea how you derived that from what I posted.
I think most people in this thread would agree, and several of them have tried to help you. Maybe you should try to understand what they're saying instead of getting defensive and angry.
Not if you're taking 1000 W per minute, you're not.
Those are called units.
Wrong. A joule (J) is defined as the work required to produce 1 Watt (W) of power for 1 second (s) or, to put it mathematically:
1 J = 1 W * s, therefore 1 J/s = 1 W
So when you say 1000 W per min, you're dividing the number of Watts by 60 seconds, not multiplying by 60 seconds. Which gives a totally different answer than the one you think you've found.
Please read up on dimensional analysis and try to understand how to apply the correct units to your calculations.
Again, if you understood dimensional analysis, you wouldn't be asking this question.
yea you gotta speak english to me
I am.
are you saying that 1000 min is a kWh?
No. I have no idea how you derived that from what I posted.
i agree there has to be something i am missing here
I think most people in this thread would agree, and several of them have tried to help you. Maybe you should try to understand what they're saying instead of getting defensive and angry.
even if i use the online conversions i get 60,000 joules
Not if you're taking 1000 W per minute, you're not.
i honestly do not know what you are meaning with the symbols
Those are called units.
you say j/s but the unit j already dictates a set time of 1 sec so there is no need to reference time again.
Wrong. A joule (J) is defined as the work required to produce 1 Watt (W) of power for 1 second (s) or, to put it mathematically:
1 J = 1 W * s, therefore 1 J/s = 1 W
So when you say 1000 W per min, you're dividing the number of Watts by 60 seconds, not multiplying by 60 seconds. Which gives a totally different answer than the one you think you've found.
Please read up on dimensional analysis and try to understand how to apply the correct units to your calculations.
do you mean 60000 to the second power? cause that is incorrect i believe
Again, if you understood dimensional analysis, you wouldn't be asking this question.
a reply to: Arbitrageur
I divided 13.17/15.91. I figured that would give the minimum efficiency (overall - of every conversion process involved) required to pull it off.
I divided 13.17/15.91. I figured that would give the minimum efficiency (overall - of every conversion process involved) required to pull it off.
originally posted by: pteridine
originally posted by: deadeyedick
originally posted by: pteridine
a reply to: deadeyedick
Look up Darwin Awards. Because you don't know what you are doing, you should be careful lest you fill up your garage with chlorine trying to see if the water bonds change energy with dissolved salt.
duh
it is a shame that you think i would not know what you are talking about even after i commented about it. I have done stupid stuff before like ignite 5gal. of browns gas at 30 psi just to see if it was true that there is power in what i was doing.
what is more a shame is the fact the claim was made that nothing can loosen the hydrogen bonds and it gets turned around to try to make it seem as though i am stupid and deserve a dumbass award for citing references that prove the claim false.
eta
to ignite the bucket of browns gas i used a hundred ft. water hose hooked up to the bucket that my output from the cells was hooked to. i had a regular water nozzle hooked up. anyhow it was quite beautiful to see water droplets form in the air as the whole thing seemed to implode on itself with about the loudest bang i have heard at that range.
Hydrogen bonding is something different; it is a dipole-dipole interaction and the reason why water has such a high relative boiling point. I didn't say you were stupid; I said that you didn't know what you were doing. The 100' hose full of an oxygen hydrogen mix proves my point.
Electrolyzing salt water because you thought that salt would weaken the hydrogen-oxygen bond would generate chlorine and could ruin your day and maybe your lungs making you eligible for the Darwin Awards. Remember to use flash arrestors when playing with stoichiometric mixtures of oxygen and hydrogen. Have fun playing but be careful.
What do you think you've proven false?
actually flash arrestors is what started the garden hose being used in the first place. Guess what i discovered? With hho there is no spark arrestor made that i have found even in welding supply stores that will work with browns gas. The only safe way is to either keep any storage of the gas to a minimum so it it ignites it will not amount to much or the best way is to bubble the gas through water and that does not work very well with pressure.
All in all the best answer is to not use hho under pressure because spark arrestors do not work because we are not just dealing with a flame but also implosion and extremely fast burn rates. You claim the use of the hose was not smart but you are wrong there also cause at that point my goal was to watch an explosion and the distance gained by the hose made things safe.
The main take away from my hands on expierence is that engines need to run on gasoline vapors enriched with browns gas. because what happens with browns gas alone is just too fast for most engines to run. the energy is burnt and gone before any force can be applied to the crank in most cases. the best i got my truck to run on the stuff was by feeding my exhaust into the intake. now there is a darwin award for you
edit on 12-1-2015 by
deadeyedick because: (no reason given)
a reply to: DenyObfuscation
where did you get 3.658 kWh, and how did you figure that 112g h2 was 15.91 MJ?
Without seeing your sources I guessed that the origin of the discrepancy is in the ratio between 237 and 286 kJ figures I cited, but if you want to post sources for your figures I can try to figure out if it's that or something else.
where did you get 3.658 kWh, and how did you figure that 112g h2 was 15.91 MJ?
Without seeing your sources I guessed that the origin of the discrepancy is in the ratio between 237 and 286 kJ figures I cited, but if you want to post sources for your figures I can try to figure out if it's that or something else.
a reply to: iterationzero
1 J/s = 1 W
1000J/s = 1000w
that is 1000w per second times 60 seconds in any diminsion
1 J/s = 1 W
1000J/s = 1000w
that is 1000w per second times 60 seconds in any diminsion
a reply to: Arbitrageur
That's in the OP, and I thought I saw that exact number somewhere else. Can't find it right now. (edit- it's on this page you linked earlier www.aardvark.co.nz...) Also saw 3.7 kWh on a physics type forum.
Bedlam posted 141.9 kJ/g for hydrogen. I'll edit to add link
www.abovetopsecret.com...
ETA: Using Bedlam's figure it actually works out to about 15.89 MJ. The 15.91 came from the power / energy calculator
where did you get 3.658 kWh
That's in the OP, and I thought I saw that exact number somewhere else. Can't find it right now. (edit- it's on this page you linked earlier www.aardvark.co.nz...) Also saw 3.7 kWh on a physics type forum.
and how did you figure that 112g h2 was 15.91 MJ?
Bedlam posted 141.9 kJ/g for hydrogen. I'll edit to add link
www.abovetopsecret.com...
edit on 12-1-2015 by DenyObfuscation because: (no reason given)
ETA: Using Bedlam's figure it actually works out to about 15.89 MJ. The 15.91 came from the power / energy calculator
edit on 12-1-2015 by DenyObfuscation because: (no reason given)
edit on 12-1-2015 by DenyObfuscation
because: (no reason given)
a reply to: deadeyedick
Why dimensional analysis is important, part 97 in a seemingly never ending series...
When you say "1000w per second", you're effectively saying 1000 W/s. If:
1 J/s = 1 W
then:
1 W/s = 1 J/s^2
then:
1000 W/s = 1000 J/s^2 and 60,000 W/s (or 1000 W/min) = 60,000 J/s^2.
In other words:
1000 W/s != 60,000 J
If you're units aren't equivalent on either side of the equals sign, you're wrong. It's really that simple.
Why dimensional analysis is important, part 97 in a seemingly never ending series...
1 J/s = 1 W
1000J/s = 1000w
that is 1000w per second times 60 seconds in any diminsion
When you say "1000w per second", you're effectively saying 1000 W/s. If:
1 J/s = 1 W
then:
1 W/s = 1 J/s^2
then:
1000 W/s = 1000 J/s^2 and 60,000 W/s (or 1000 W/min) = 60,000 J/s^2.
In other words:
1000 W/s != 60,000 J
If you're units aren't equivalent on either side of the equals sign, you're wrong. It's really that simple.
a reply to: deadeyedick There are several "Brown's Gas" websites that will be happy to sell you flash arrestors. Youtube
provides video demos of same.
Bedlam's figure is accurate for the amount of electrical energy required for dissociation (and it correlates with the figure I've been using). However there is more energy required than I have been citing (or in Bedlams figure), as shown in this diagram:
originally posted by: DenyObfuscation
a reply to: Arbitrageur
where did you get 3.658 kWh
That's in the OP, and I thought I saw that exact number somewhere else. Can't find it right now. Also saw 3.7 kWh on a physics type forum.
and how did you figure that 112g h2 was 15.91 MJ?
Bedlam posted 141.9 kJ/g for hydrogen. I'll edit to add link
www.abovetopsecret.com...
hyperphysics.phy-astr.gsu.edu...
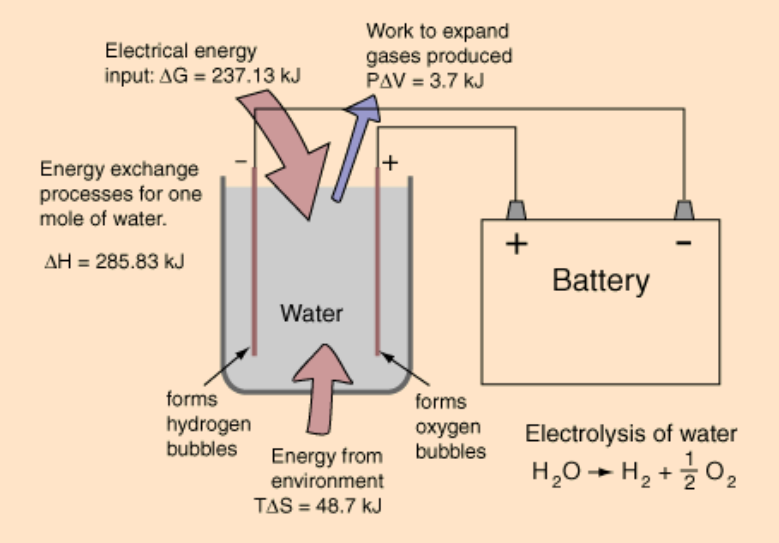
See the top red arrow pointing in for "electrical energy input"? That's what Bedlam's figure represents, and it's correct, but that won't complete the electrolysis without some help. You also need the other input shown by the bottom red arrow that says "energy from environment". Bedlam's figure doesn't include that, nor do my figures. In my case I left that out because I was doing theoretical best case scenarios on electrolysis efficiency, where you might have an external heat source.
But if you don't have an external heat source, you need to add that, in which case Bedlam's number and my numbers are too low. The OP didn't cite a source but I'm guessing if the number is accurate, it includes both the top and the bottom red arrow, so if you compare that to Bedlam's number which only includes the top red arrow, you're not comparing apples and apples.
edit on 12-1-2015 by Arbitrageur because: clarification
a reply to: Arbitrageur
I'm seeing the 141.9 kJ/g as being the energy content of the H2, as in how many joules combustion would yield. Is that incorrect?
I'm seeing the 141.9 kJ/g as being the energy content of the H2, as in how many joules combustion would yield. Is that incorrect?
I am astonished that this thread made five pages.
With luck there'll be a nice big explosion soon.
With luck there'll be a nice big explosion soon.
edit on 12/1/15 by Astyanax because: of particularities.
originally posted by: deadeyedick
a reply to: Bedlam
lol
it is quite simple
1w=1joule per second
1000w=1000joules per second
1000w per min=60,000 joules
When you move the "per second" to the left with the 1000W, it flips to the top and multiplies instead of dividing. Basic. Algebra.
originally posted by: deadeyedick
Saltwater has a lower freezing point than pure water.
Do you get it? The weaker the bond the lower the freezing point.
That's not the reason for freezing point depression, which is a colligative property that alters as the solute leaves the solvent for the solid mass. In other words, the mixture's ratio changes as freezing occurs, it pretty much always lowers the freezing point and raises the boiling point.
Chemistry 101.
originally posted by: deadeyedick
a reply to: Arbitrageur
how is 120watts not a specified amount of energy and time
1w=1joule per second
120 Watts is power. Not energy, not time.
1 W = 1 Joule/sec. This is correct. Now, this is with power on both sides of the equation. Remember, that = sign means both sides are literally the same. Not sorta related.
Joules are energy. Energy is power * time. So, by dividing Joules by seconds, you get a power number. (power*time)/time = power. So, Joules are energy, but a Joule/sec is a power number, like a Watt. One Joule/second is a Watt, just like the equation says.
Now. If you move the seconds in the right denominator over to the left, it'll mathemagically change both sides to energy.
But you can't do it your way, so that 1W = 1Joule/sec ===> 1Watt/sec = 1 Joule, because that algebra is wrong.
What you get is more like 1WattSecond = 1Joule. Now, you got energy terms on both sides. A Watt Second is energy. A Watt/second is nothing, because it's not a unit. It's a very basic algebra mistake you're making.
That's what many published figures for energy content say, but realistically that's not what you'd get in combustion unless your output of the combustion process was 100% liquid water, and it's not going to be 100% liquid water, is it? That's what that figure is based on, and it's called the HHV (Higher Heating Value). The LHV (Lower Heating Value) for hydrogen is only 119.93 kJ/g, which is a more realistic figure if the product of combustion is water vapor, instead of liquid water. Source (see pdf page 21 Table 1-3).
originally posted by: DenyObfuscation
I'm seeing the 141.9 kJ/g as being the energy content of the H2, as in how many joules combustion would yield. Is that incorrect?
Heat of Combustion
LHV calculations assume that the water component of a combustion process is in vapor state at the end of combustion, as opposed to the higher heating value (HHV) (a.k.a. gross calorific value or gross CV) which assumes that all of the water in a combustion process is in a liquid state after a combustion process.
I figured out where the OP number came from and now I can say I was sort of right about where the 82.7% came from in that one figure included only the top arrow and the other figure included both the top and bottom red arrows, but I had those reversed, so to correct what I said before, it's Bedlam's figure that includes both top and bottom red arrows, and the OP figure that includes only the top red arrow.
Here's how the numbers work out going from water, to HHO, and back to water again assuming 1000g of water (1 liter if density is 1.0):
convert 1000g liquid water to HHO
Apply 13.172 MJ (which is 237.13 kJ/mole x 55.55 moles, which is equivalent to 3.659kWh) of electrical energy + 2.705 MJ (which is 48.7 kJ/mole x 55.55 moles, which is equivalent to ~0.751kWh) other (thermal) energy to 1 liter of water to get HHO gas
13.172 MJ + 2.705 MJ = 15.9 MJ (in the diagram I posted, this is top arrow plus bottom arrow = total)
In the diagram I posted, the 13.172 MJ (aka ~3.659kWh which is the OP figure) is only the top red arrow. You have to add the other 2.705MJ to get the total energy required, which is missing from the OP figure.
convert 1000g HHO back to water
As we discussed there is 112g Hydrogen in 1000g of HHO. Multiply 112g by 141.86 kJ/g and you get 15.9MJ
So you put 15.9MJ in and you get 15.9MJ out if there are zero losses anywhere and if the result of the combustion is liquid water, which are obviously totally unrealistic assumptions.
edit on 13-1-2015 by Arbitrageur because: clarification
a reply to: deadeyedick
Listen, my dear dick,
All these clever people don't seem to be able to make you understand how horribly you have gone wrong, so let me, in my stupid mind-firmly-closed way, give it a shot.
What you are doing with joules and watts? Basically, it's exactly like getting your miles mixed up with your miles per hour.
It's that basic. That kindergarten. That sad. Have a nice day.
Listen, my dear dick,
All these clever people don't seem to be able to make you understand how horribly you have gone wrong, so let me, in my stupid mind-firmly-closed way, give it a shot.
What you are doing with joules and watts? Basically, it's exactly like getting your miles mixed up with your miles per hour.
It's that basic. That kindergarten. That sad. Have a nice day.
originally posted by: GetHyped
I'll keep an eye on the obituaries for further updates.
To put it into perspective...
Man owes a great debt to the scientists on this list; all of them died or were injured in their pursuit of knowledge. The advances they have all made to science are extraordinary and many of them paved the way for some of man’s greatest discoveries and inventions.
Top 10 scientists killed or injured during experimentation.
10: Karl Scheele
Died from tasting his discoveries, eventually died from Mercury poisoning, after making discoveries we all rely upon today.
9: Jean-Francois De Rozier
First victim of an air crash. Jean-Francois took the first manned free flight in a balloon. Unfortunately, he died trying to cross the English channel in a balloon, but not before he had conclusively demonstrated Human flight was a reality.
8: Sir David Brewster
Nearly blinded, during a chemical experiment. Working in Optics, inventing the kaleidoscope, he was almost blinded by his work.
7: Elizabeth Ascheim
Killed by X-Rays. She set up the first x-ray lab in San Francisco, during experimentation, she was exposed to lethal doses of radiation and died of widespread cancer.
6: Alexander Bogdanov
Killed himself with blood.Pioneering blood transfusions, he experimented on himself. Unfortunately, he used blood infected with parasites and bacteria, which eventually ended up killing him.
5: Robert Bunsen
Blinded himself in one eye. Everyone has at least heard of and most have used a Bunsen burner in a Chemistry class or lab.
Bunsen started his career in organic chemistry and almost died twice of arsenic poisoning. He went on to lose the sight in an eye, due to a chemical explosion.
4: Sir Humphrey Davy
A catalogue of disasters. He was fired from his job at an apothecary because he caused too many explosions, and took up the field of chemistry. He took to inhaling the various gasses he was dealing with. Fortunately, this led to the discovery of anaesthetic gases like Nitrous Oxide. Due to these rigours, he was an invalid for the latter half of his life.
3: Michael Faraday
Suffered chronic poisoning. Faraday suffered damage to his eyes in a nitrogen chloride explosion. He spent the remainder of his life suffering chronic chemical poisoning.
2: Marie Curie
Died of radiation exposure. Her constant exposure to radiation led to her contracting leukemia and she died in 1934.
But not before winning two Nobel prizes in separate fields (only person to have achieved this feat) and laying ground work that still benefits Humanity today.
1: Galileo Galilei
Blinded himself. He was fascinated with the sun and spent many hours staring at it – leading to extreme damage to his retinas, causing near total blindness. His scientific contribution is immeasurable.
The bottom line?
Science isn't always clean and safe, to become a pioneer and hopefully leave a lasting and positive legacy of increasing our understanding and advancement, one sometimes has to take a walk on the wild side and venture into the unknown and of course, be prepared to take risks...just as every scientific giant had done themselves.
My opinion is we should be cautious, we ought to be mindful of safety, but to hunt for systems and processes that may one day lead to a cheap, clean, abundant and safe energy technology for our species, and reverse environmental damage wreaked upon our little world by the overuse of fossil fuels, a little risk is a very good trade off.
edit on 13-1-2015 by MysterX because: typo
new topics
-
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents: 21 minutes ago -
Australian PM says the quiet part out loud - "free speech is a threat to democratic dicourse"...?!
New World Order: 1 hours ago -
Ireland VS Globalists
Social Issues and Civil Unrest: 1 hours ago -
Biden "Happy To Debate Trump"
Mainstream News: 2 hours ago -
RAAF airbase in Roswell, New Mexico is on fire
Aliens and UFOs: 2 hours ago -
What is the white pill?
Philosophy and Metaphysics: 3 hours ago -
Mike Pinder The Moody Blues R.I.P.
Music: 4 hours ago -
Putin, Russia and the Great Architects of the Universe
ATS Skunk Works: 7 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 17 hours ago, 35 flags -
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 12 hours ago, 20 flags -
Mike Pinder The Moody Blues R.I.P.
Music: 4 hours ago, 7 flags -
What is the white pill?
Philosophy and Metaphysics: 3 hours ago, 5 flags -
Biden "Happy To Debate Trump"
Mainstream News: 2 hours ago, 5 flags -
Australian PM says the quiet part out loud - "free speech is a threat to democratic dicourse"...?!
New World Order: 1 hours ago, 4 flags -
RAAF airbase in Roswell, New Mexico is on fire
Aliens and UFOs: 2 hours ago, 4 flags -
Putin, Russia and the Great Architects of the Universe
ATS Skunk Works: 7 hours ago, 3 flags -
Ireland VS Globalists
Social Issues and Civil Unrest: 1 hours ago, 2 flags -
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents: 21 minutes ago, 1 flags
active topics
-
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 286 • : cherokeetroy -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics • 98 • : Vermilion -
Biden "Happy To Debate Trump"
Mainstream News • 31 • : theatreboy -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 84 • : YourFaceAgain -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 33 • : theatreboy -
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents • 3 • : chiefsmom -
Hate makes for strange bedfellows
US Political Madness • 52 • : YourFaceAgain -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 697 • : Thoughtful3 -
So this is what Hamas considers 'freedom fighting' ...
War On Terrorism • 265 • : YourFaceAgain -
Ireland VS Globalists
Social Issues and Civil Unrest • 6 • : TimBurr
