It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
a reply to: PeterMcFly
I understand.
I don't understand your "what happens" as it applies to radiative forcing within the atmosphere. I'm also not sure what significance 10 microns has.
That's why I asked.
I understand.
I don't understand your "what happens" as it applies to radiative forcing within the atmosphere. I'm also not sure what significance 10 microns has.
That's why I asked.
a reply to: Phage
If you increase emissivity of the atmosphere at specific wavelength, I understand the "opacification" will reflect back LWIR toward Earth, but the atmosphere, having gained emissivity (due to absorbitivity) will cool itself more due to black body emission toward space (grey body if you prefer).
I.E.: Try to measure equivalent temperature using LWIR of a gold mirror (emissivity= 0, reflectivity= 1). Now try it with a charcoal piece.
If you increase emissivity of the atmosphere at specific wavelength, I understand the "opacification" will reflect back LWIR toward Earth, but the atmosphere, having gained emissivity (due to absorbitivity) will cool itself more due to black body emission toward space (grey body if you prefer).
I.E.: Try to measure equivalent temperature using LWIR of a gold mirror (emissivity= 0, reflectivity= 1). Now try it with a charcoal piece.
a reply to: PeterMcFly
Makes no sense.
having gained emissivity (due to absorbitivity)
How? If more infrared is being returned because of the increase in CO2 "coins".
will cool itself more due to black body emission toward space (grey body if you prefer)
Yes, the charcoal will get warmer because it is absorbing infrared radiation. Just like the surface of the Earth gets warming when there is more infrared radiation to absorb (being re-emitted by CO2 back toward the surface). That's pretty much the basic idea. Without CO2 the Earth would cool very rapidly at night. The more CO2, the slower that happens.
Try to measure equivalent temperature using LWIR of a gold mirror (emissivity= 0, reflectivity= 1). Now try it with a charcoal piece.
Not really. CO2 most strongly absorbs (and emits) at three wavelengths; about 3, 5, and 15 microns.
It has a lot significance, the whole concept of "Greenhouse effect" is based upon it...
edit on 1/31/2015 by Phage because: (no reason given)
a reply to: Phage
CO2 absorbs heat as vibrational and rotational energy, as most molecules do.
The heat absorbed by CO2 becomes motion, not a higher quantum state. Most of the Infra Red energy is transferred to the rest of the atmosphere kinetically. The warmer, faster, more energetic molecules will evolve higher into the atmosphere, which is convection, and while doing so radiate out the additional energy as black body radiation.
CO2 at .04% is no where near as dense as clouds. Water absorbs infrared across a wider spectrum than CO2.
Also there is probably a increase in air pressure due to the weather fact that solutions of different density don't mix.
The increase in pressure will lead to an increase in temperature, if the volume remains constant, which the clouds would contribute to.
gone till tomorrow
The way it works is this; The problem isn't the "heat" that CO2 absorbs, the problem is that it absorbs infrared radiation. For a little while. When it does this, it causes an electron to jump to a higher energy level.
CO2 absorbs heat as vibrational and rotational energy, as most molecules do.
While in many lasers the laser process involves the transition of atoms between different electronic energy states, as described in the model above, this is not the only mechanism that can result in laser action. For example, there are many common lasers (e.g., dye lasers, carbon dioxide lasers) where the laser medium consists of complete molecules, and energy states correspond to vibrational and rotational modes of oscillation of the molecules.
en.wikipedia.org...
The heat absorbed by CO2 becomes motion, not a higher quantum state. Most of the Infra Red energy is transferred to the rest of the atmosphere kinetically. The warmer, faster, more energetic molecules will evolve higher into the atmosphere, which is convection, and while doing so radiate out the additional energy as black body radiation.
Ever notice how on an overcast night it is often warmer than on a clear night? That's because the clouds are reflecting infrared radiation back to the surface. Same principle except that clouds are a bit more directional than CO2 molecules.
CO2 at .04% is no where near as dense as clouds. Water absorbs infrared across a wider spectrum than CO2.
Also there is probably a increase in air pressure due to the weather fact that solutions of different density don't mix.
The increase in pressure will lead to an increase in temperature, if the volume remains constant, which the clouds would contribute to.
gone till tomorrow
a reply to: Semicollegiate
Heat is not the same thing as electromagnetic radiation. Heat is, as you have pointed out, mechanical energy. CO2 does absorb heat when other molecules bump into it. But it also absorbs (and re-emits) electromagnetic radiation.
CO2 absorbs heat as vibrational and rotational energy, as most molecules do.
We aren't talking about heat but you are correct about the quantum state.
The heat absorbed by CO2 becomes motion, not a higher quantum state.
In the infrared, yes. And some of that radiation will be directed back toward the surface. The more CO2, the more will end up heading back to the surface instead of into space.
and while doing so radiate out the additional energy as black body radiation.
I didn't say that CO2 is as dense as clouds. I said that both display the same characteristic, reflecting infrared back to the surface. The more clouds, the more infrared. The more CO2, the more infrared.
CO2 at .04% is no where near as dense as clouds. Water absorbs infrared across a wider spectrum than CO2.
edit on 1/31/2015 by Phage because: (no reason given)
a reply to: Phage
No problem then, simply extend the concept to other spectral bands, no difference... Kirchhoff's law of thermal radiation stay the same...
Not really. CO2 most strongly absorbs (and emits) at three wavelengths; about 3, 5, and 15 microns.
No problem then, simply extend the concept to other spectral bands, no difference... Kirchhoff's law of thermal radiation stay the same...
edit on 2015-1-31 by PeterMcFly because: (no reason given)
a reply to: PeterMcFly
Maybe if you tried to explain how "having gained emissivity (due to absorbitivity)" in more that one sentence I could understand your point better and how it contradicts my "coin" analogy.
Yes.
Are you sure you grasp 'Kirchhoff's law of thermal radiation'?
Maybe if you tried to explain how "having gained emissivity (due to absorbitivity)" in more that one sentence I could understand your point better and how it contradicts my "coin" analogy.
Yes, more absorbers/emitters means more radiation will be directed back to the surface. Still not clear why you thought 10 microns was important enough to specify.
No problem then, simply extend the concept to other spectral bands, no difference
edit on 1/31/2015 by Phage because: (no reason given)
a reply to: PeterMcFly
Ok. So, the re-emitted energy is well trained and only goes out to space.
Got it.
Ok. So, the re-emitted energy is well trained and only goes out to space.
Got it.
originally posted by: Phage
a reply to: Semicollegiate
No. Climatologists do not say that increasing levels of CO2 will heat the planet by absorbing heat. Climatologists (and physicists) say that increasing levels of CO2 will heat the planet by reducing the amount of energy sent back into space.
Climate alarmists cry that 0.04% of the atmosphere is going to heat up the planet by absorbing 8% of the ambient (i.e. room temperature) heat.
The way it works is this; The problem isn't the "heat" that CO2 absorbs, the problem is that it absorbs infrared radiation. For a little while. When it does this, it causes an electron to jump to a higher energy level. But that electron returns to its orginal state after a bit and when it does it releases a photon of infrared radiation (just like what it absorbed). The problem is, where does that infrared radiation go?
Think about it like flipping a coin. There is a 50% chance that a given CO2 molecule will re-emit infrared radiation into space instead of back to Earth. (above the horizon or below it). Let's say we don't have any coins. No CO2 in the atmosphere. Outgoing radiation just keeps on going out. 100% of it. Earth's atmosphere is very cold.
Now let's add one "coin" worth of CO2. What happens? 50% chance that you'll get "tails". Earth gets warmer because the amount of radiation leaving is no longer the same as the amount of radiation incoming. Half of it is coming back to the surface.
Now let's add another "coin". What happens? With 2 coins the odds are 75% that you'll get at least one tail. Earth gets warmer still.
With 3 coins the odds are 87% that you'll get a tail. Earth gets warmer still.
The more CO2 there is in the atmosphere, the more radiation will be re-emitted downward. But, you say, more will also be re-emitted upward. But more cannot be re-emitted upward. To understand why, go back to the no coin situation. The amount of escaping radiation cannot increase beyond 100% but the amount of returning radiation can increase from 0% and does. When 100% of the energy escapes, its cold. When 90% escapes it's a bit warmer. When 75% escapes it's warmer still. When the balance (where ever it may be) changes, the temperature of the Earth changes. Increasing GHGs is one thing that changes the balance. Increasing GHGs means that more infrared radiation stays in the atmosphere and less leaves. The concept is called radiative forcing, not heat absorption.
Ever notice how on an overcast night it is often warmer than on a clear night? That's because the clouds are reflecting infrared radiation back to the surface. Same principle except that clouds are a bit more directional than CO2 molecules.
Just to further on this excellent explanation:
99% of the atmosphere - Oxygen, Nitrogen and Argon - is effectively invisible to this process. That’s because these monatomic and diatomic molecules do not vibrate at infrared wavelengths the way triatomic CO2 and other GHGs do. This effect is easily demonstrated in the lab using spectral analysis, but here’s a more basic youtube video that helps visualize it:
So when someone says CO2 is “only 0.04%” of the atmosphere, this is entirely irrelevant. Because CO2 makes up a much larger proportion (up to 25%) of the Greenhouse Effect, and that’s what matters.
And for anyone actually interested in the math – there is plenty of first principles algebra out there that explains how we know the Greenhouse Effect already warms the earth naturally by ~33 C, here for example:
www.atmos.washington.edu...
a reply to: Phage
OK, then I will try another analogy, in electro-optics, there are two regime that shall not be mixed together; reflective and emissive regime.
The fog analogy is usefull to understand reflective regime. It's match well with the coin analogy because the atmosphere act as scatterer (if you want reflection). This is very applicable at visible and NIR wavelength. But at wavelength we talk for 'green house' effect we are in emissivity regime (LWIR).
All the opacifier (the fog) act as black body emitter, not just the lower level that will reflect energy toward Earth. But every molecule will emit by black body mecanism. In reflective regime (visible or NIR), this is not possible since air is not hot enough to produce significant emission at visible or NIR, but at around 20degC the emisssion max is around 10 or 12 um, that's the green house effect.
OK, then I will try another analogy, in electro-optics, there are two regime that shall not be mixed together; reflective and emissive regime.
The fog analogy is usefull to understand reflective regime. It's match well with the coin analogy because the atmosphere act as scatterer (if you want reflection). This is very applicable at visible and NIR wavelength. But at wavelength we talk for 'green house' effect we are in emissivity regime (LWIR).
All the opacifier (the fog) act as black body emitter, not just the lower level that will reflect energy toward Earth. But every molecule will emit by black body mecanism. In reflective regime (visible or NIR), this is not possible since air is not hot enough to produce significant emission at visible or NIR, but at around 20degC the emisssion max is around 10 or 12 um, that's the green house effect.
originally posted by: Semicollegiate
a reply to: Phage
The heat absorbed by CO2 becomes motion, not a higher quantum state. Most of the Infra Red energy is transferred to the rest of the atmosphere kinetically.
Nope.
The IR absorbed through vibrational and rotational mechanics is simply re-emitted as a photon again because of the resulting oscillation of the molecule's dipole. This is straight up electromagnetics.
scied.ucar.edu...
a reply to: PeterMcFly
You know you can try to rationalize it away however you like (I'm completely lost by your explanation too), but on the first page of this thread I already posted some real world observations that demonstrate how you're just plain wrong:
You know you can try to rationalize it away however you like (I'm completely lost by your explanation too), but on the first page of this thread I already posted some real world observations that demonstrate how you're just plain wrong:
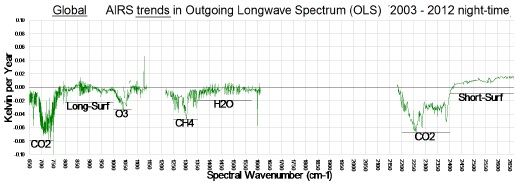
Exactly. Satellite data doesn't just show increases in temperature, it also shows heat being trapped at the exact wavelengths CO2 is known to do so. We can literally see man-made global warming happening from space:
Ground based measurements also capture this infrared radiation bouncing back to Earth:
Evidence for man made global warming is absolutely overwhelming. But the denialists just choose to ignore all this, or pretend it's fake when they have no other recourse.
a reply to: mc_squared
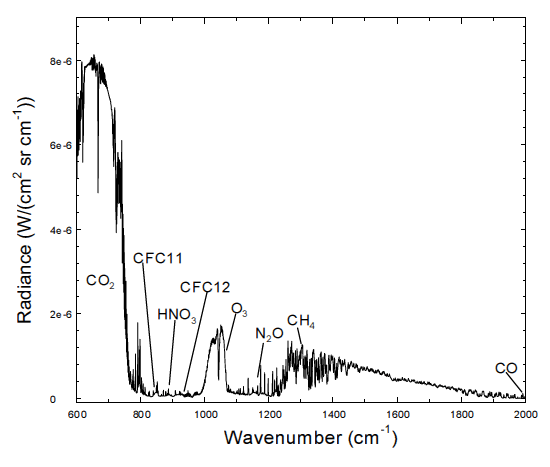
Please stop trying to mystify people with your cm-1 and post your graph using plain old nm and um ...
Please stop trying to mystify people with your cm-1 and post your graph using plain old nm and um ...
a reply to: PeterMcFly
Dear lord, yeah you sound really qualified to be lecturing others here on atmospheric physics.
WAVENUMBER/WAVELENGTH CONVERTER
Dear lord, yeah you sound really qualified to be lecturing others here on atmospheric physics.
WAVENUMBER/WAVELENGTH CONVERTER
originally posted by: PeterMcFly
a reply to: Phage
If you increase emissivity of the atmosphere at specific wavelength, I understand the "opacification" will reflect back LWIR toward Earth, but the atmosphere, having gained emissivity (due to absorbitivity) will cool itself more due to black body emission toward space (grey body if you prefer).
Indeed, which is why the stratosphere is cooling while the surface and oceans are warming.
www.realclimate.org...
The climatologists do NOT have the mechanism of greenhouse effect wrong. They've been studying this for 50 years and is the most certain part of this business.
Phage is right on this: the effect comes from absorbing, and then re-radiating isotropically infrared which previously would be emitted only to space. The net result is that the equilibrium temperature on the surface is raised.
About opacity and whether it was 'saturated' already in pre-industrial times: no.
How the science was developed and how it works: www.realclimate.org...
Besides, behold Venus.
edit on 31-1-2015 by mbkennel because: (no reason given)
new topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 3 hours ago -
Maestro Benedetto
Literature: 5 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 5 hours ago -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 9 hours ago -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 9 hours ago -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 10 hours ago -
The functionality of boldening and italics is clunky and no post char limit warning?
ATS Freshman's Forum: 11 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 3 hours ago, 25 flags -
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest: 15 hours ago, 9 flags -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media: 17 hours ago, 8 flags -
Weinstein's conviction overturned
Mainstream News: 13 hours ago, 8 flags -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics: 15 hours ago, 8 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 12 hours ago, 7 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 10 hours ago, 7 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 9 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 12 hours ago, 5 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 9 hours ago, 4 flags
active topics
-
Truth Social goes public, be careful not to lose your money
Mainstream News • 130 • : Astyanax -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs • 11 • : AwakeNotWoke -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 15 • : AwakeNotWoke -
Is AI Better Than the Hollywood Elite?
Movies • 13 • : Justoneman -
Hate makes for strange bedfellows
US Political Madness • 47 • : 19Bones79 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 689 • : daskakik -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 265 • : Astrocometus -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 27 • : ToneD -
Reason of the Existence
The Gray Area • 21 • : BingoMcGoof -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics • 85 • : Sookiechacha