It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
Dummies Guide to EASY silver bullion refining at home as a long term precious metal investment
page: 1share:
As informed ATS mermbers, I'm sure you're all perfectly aware of the extreme volatility in the current global financial market and even more aware
of how our money buys less and less and less as the years go by. What's also very apparent to those that watch the precious metals market e.g. silver
and gold, that contrary to the "value" of paper based currency which has been continuously decreasing, that the "value" of silver and gold has
been on a continual increase.
Here's a couple of graphs showing the improvement of silver over the last 30 and 60 days.
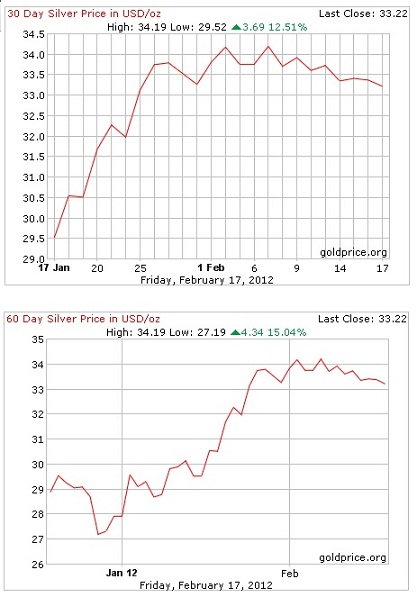
Now over the last few years, I've been taking scrap, old, unwanted and broken items containing silver and refining them in such a way as to end up with essentially 99.9% silver bullion. Originally this was nothing more than a home hobby of sorts but with the value of silver and gold bullion apparently on an increasing trend, what started out as just a hobby has now become a means of creating silver bullion as a long term investment. I get quite a kick in checking the silver markets and watching the value of my silver slowly rise
And so I figured that now would be a perfect opportunity to share my experience in silver bullion creation at home with other ATS members who may have an interest.
Not only will you end up being able to create pure silver bullion of your own, but you'll also see how ridiculously simple and easy the process is and that the majority of equipment is easily obtainable and very inexpensive.
In fact, over the years I've simplified the entire process so that anyone can do it. So why not read this tutorial and perhaps give it a go yourself.
If there's sufficient positive response, I'll consider doing a similar thread and show once again how ridiculously easy it is to process and refine old, scrap, broken, etc gold items into 99.9% 24k gold ingots at home using essentially the same basic and inexpensive equipment used to produce silver bullion.
Just to whet your appetite, here's a pic of some pure silver bullion "buttons" that I've made:

and here's a pic of a 24k gold button (8 gms) I made earlier today ... and worth approx. $US443 at todays market rates ... hopefully next week will be worth a bit more

Interested ?
Ok, let's start processing silver !
We need some basic equipment which consists of a plastic water spray bottle, a plastic spoon, a plastic "swizzle" stick, a plastic "turkey baster" pipette and a small plastic funnel.
All these items were purchased at one of those "cheap" stores for just a few dollars only.

We need a couple of small glass cylinders and in my case, I used some "empty" spice jars from the kitchen. At least that's what I told my wife
Also need a small glass bowl able to stand up to boiling water and that will allow one of the spice jars to be placed upright in it. The boiling water poured into the bowl will help heat the contents of the spice jar.

We will also need some cheap disposable plastic gloves such as the ones used for food handling.
We'll also need some coffee filters. Again, both of these items were purchased at the "cheap" store for a couple of dollars.

The next item that we'll need is a bottle of 70% strength Nitric acid. This is readily obtainable from the majority of chemical supply companies which you'll have no problems in finding in your local yellow pages. A 500 ml bottle will be quite adequate.
The nitric acid is used to dissolve the silver and any other contaminating metals in the silver such as copper, etc. We need to eventually remove the contaminants leaving behind pure silver for final processing.

At this point, a couple of WARNINGS are required, so PLEASE PAY ATTENTION.
Nitric acid, by it's nature is a powerful corrosive acid and skin contact should be avoided. When working with it, you should ALWAYS be wearing those disposable plastic gloves that we purchased earlier.
Simple care, caution and common sense is all that's needed and you'll be perfectly safe. In all the years that I've been refining silver and gold for a hobby and using nitric acid, not once has there been an issue or concern. If any is spilled on a surface, just immediately rinse off with plain water.
Also, small quantities of nitrogen dioxide gas is produced so ALWAYS do the refining outside in the open air.
The motto is: "Be careful and be safe".
But let me reiterate one more time ... if you exercise common sense and basic care, this entire procedure is perfectly safe. Again, I've been doing this for quite a few years with NO mishaps whatsoever.
And to complete our refining "kit", we need someway of melting the raw silver into the shiny metal that people have lusted after for millenia
Thankfully, all we need to do is head to the nearest hardware store and get something along the lines of this badboy ... a propane powered torch. This is the one I use because it has a self-igniting switch built in, so no need to use matches to get it going. Also has a rotary button to control the strength of the flame. No problems whatsoever in finding one as they're a common handyman item and it easily melts silver and gold.

Well, thats it ... that's all the simple equipment that we need to start producing our very own 99.9% pure silver bullion and then watching it appreciate over time !
And as you can see, all of it is extremely easy to find.
As for the material containing silver that will be processed, sure you could use old bits of silver jewelry or anything else that you've got lying around with a reasonable silver content. But personally, I found the simplest way to get all my "raw silver items" is in the form of old coins containing silver and purchased on eBay. Quite often you can get some really good deals.
As an example, last week I purchased a half kilo of out of circulation Australian sixpence coins dating back to the 40's, 50's and 60's. By themselves as coins, they're worth next to nothing and don't have much intrinsic value. But each one of the coins has a 50% silver / 50% copper makeup that makes them a perfect source of silver. Sure beats trying to dig it out of the ground the hard way

Ok, for the purpose of this tutorial, lets process 2 of those coins and recover the pure silver from them.
The coins weigh in total 6 gms but because they're a 50/50 mix of silver/copper, the actual amount of silver works out to be 3 gms.
To recover the silver component, we need to completely dissolve the 2 coins in a 50/50 mixture of nitric acid and water. The amount of nitric acid required will be approximately 17mls mixed with approximately 17 mls of water. Using the plastic pipette, the water is added first followed by the nitric acid.

Continued next post ...
Here's a couple of graphs showing the improvement of silver over the last 30 and 60 days.

Now over the last few years, I've been taking scrap, old, unwanted and broken items containing silver and refining them in such a way as to end up with essentially 99.9% silver bullion. Originally this was nothing more than a home hobby of sorts but with the value of silver and gold bullion apparently on an increasing trend, what started out as just a hobby has now become a means of creating silver bullion as a long term investment. I get quite a kick in checking the silver markets and watching the value of my silver slowly rise
And so I figured that now would be a perfect opportunity to share my experience in silver bullion creation at home with other ATS members who may have an interest.
Not only will you end up being able to create pure silver bullion of your own, but you'll also see how ridiculously simple and easy the process is and that the majority of equipment is easily obtainable and very inexpensive.
In fact, over the years I've simplified the entire process so that anyone can do it. So why not read this tutorial and perhaps give it a go yourself.
If there's sufficient positive response, I'll consider doing a similar thread and show once again how ridiculously easy it is to process and refine old, scrap, broken, etc gold items into 99.9% 24k gold ingots at home using essentially the same basic and inexpensive equipment used to produce silver bullion.
Just to whet your appetite, here's a pic of some pure silver bullion "buttons" that I've made:

and here's a pic of a 24k gold button (8 gms) I made earlier today ... and worth approx. $US443 at todays market rates ... hopefully next week will be worth a bit more

Interested ?
Ok, let's start processing silver !
We need some basic equipment which consists of a plastic water spray bottle, a plastic spoon, a plastic "swizzle" stick, a plastic "turkey baster" pipette and a small plastic funnel.
All these items were purchased at one of those "cheap" stores for just a few dollars only.

We need a couple of small glass cylinders and in my case, I used some "empty" spice jars from the kitchen. At least that's what I told my wife
Also need a small glass bowl able to stand up to boiling water and that will allow one of the spice jars to be placed upright in it. The boiling water poured into the bowl will help heat the contents of the spice jar.

We will also need some cheap disposable plastic gloves such as the ones used for food handling.
We'll also need some coffee filters. Again, both of these items were purchased at the "cheap" store for a couple of dollars.

The next item that we'll need is a bottle of 70% strength Nitric acid. This is readily obtainable from the majority of chemical supply companies which you'll have no problems in finding in your local yellow pages. A 500 ml bottle will be quite adequate.
The nitric acid is used to dissolve the silver and any other contaminating metals in the silver such as copper, etc. We need to eventually remove the contaminants leaving behind pure silver for final processing.

At this point, a couple of WARNINGS are required, so PLEASE PAY ATTENTION.
Nitric acid, by it's nature is a powerful corrosive acid and skin contact should be avoided. When working with it, you should ALWAYS be wearing those disposable plastic gloves that we purchased earlier.
Simple care, caution and common sense is all that's needed and you'll be perfectly safe. In all the years that I've been refining silver and gold for a hobby and using nitric acid, not once has there been an issue or concern. If any is spilled on a surface, just immediately rinse off with plain water.
Also, small quantities of nitrogen dioxide gas is produced so ALWAYS do the refining outside in the open air.
The motto is: "Be careful and be safe".
But let me reiterate one more time ... if you exercise common sense and basic care, this entire procedure is perfectly safe. Again, I've been doing this for quite a few years with NO mishaps whatsoever.
And to complete our refining "kit", we need someway of melting the raw silver into the shiny metal that people have lusted after for millenia
Thankfully, all we need to do is head to the nearest hardware store and get something along the lines of this badboy ... a propane powered torch. This is the one I use because it has a self-igniting switch built in, so no need to use matches to get it going. Also has a rotary button to control the strength of the flame. No problems whatsoever in finding one as they're a common handyman item and it easily melts silver and gold.

Well, thats it ... that's all the simple equipment that we need to start producing our very own 99.9% pure silver bullion and then watching it appreciate over time !
And as you can see, all of it is extremely easy to find.
As for the material containing silver that will be processed, sure you could use old bits of silver jewelry or anything else that you've got lying around with a reasonable silver content. But personally, I found the simplest way to get all my "raw silver items" is in the form of old coins containing silver and purchased on eBay. Quite often you can get some really good deals.
As an example, last week I purchased a half kilo of out of circulation Australian sixpence coins dating back to the 40's, 50's and 60's. By themselves as coins, they're worth next to nothing and don't have much intrinsic value. But each one of the coins has a 50% silver / 50% copper makeup that makes them a perfect source of silver. Sure beats trying to dig it out of the ground the hard way

Ok, for the purpose of this tutorial, lets process 2 of those coins and recover the pure silver from them.
The coins weigh in total 6 gms but because they're a 50/50 mix of silver/copper, the actual amount of silver works out to be 3 gms.
To recover the silver component, we need to completely dissolve the 2 coins in a 50/50 mixture of nitric acid and water. The amount of nitric acid required will be approximately 17mls mixed with approximately 17 mls of water. Using the plastic pipette, the water is added first followed by the nitric acid.

Continued next post ...
Continued from previous post ...
Almost immediately, you'll see a bubbling reaction begin and the liquid start to change colour from clear to a very pretty blue shade. This blue colour is due to the copper in the coins reacting with the nitric acid to produce copper nitrate.
Also, brown vapours of nitrogen dioxide will also form.
WARNING Nitrogen dioxide is a NASTY, NASTY, NASTY gas and under no circumstances should you breathe it in. As mentioned earlier, do all this refining in the open air and you'll be perfectly ok.

To speed up the reaction, we can place the glass tube into a bath of boiling hot water. As the bath water cools, replace with fresh, boiling water.

Now, we sit back and let the reaction continue until both coins have been completely dissolved leaving behind just a brilliant blue liquid.

When the coins are completely dissolved, we need to dilute this blue liquid a bit so add an equal volume of water.
Next, we need to remove any sediment by pouring this blue liquid through a filter into the 2nd of our glass jars.
Here's how to create a suitable filter using one of the coffee filters.
1. Use a round lid to mark out a circular pattern on the filter.
2. Cut out the circular shape.
3. Fold the circular filter in half ... then fold in half again.
4. Carefully open up the filter and you'll find it should fit easily into the plastic funnel.

Place the funnel and filter into the opening of the 2nd (empty) glass jar and carefully pour the blue liquid into the funnel, allowing it to drip through.

By the way, you ARE wearing your plastic gloves, aren't you ? If you splash the blue liquid onto skin you'll get a harmless black discoloration that will take about 4 days to wear off. So wear your gloves !! That black discoloration is caused by silver in the blue liquid reacting to light and darkening ... the basis of how photographic film works :-)
Should have taken my own advice

Ok, we want to get every last bit of silver so once the liquid has finished running through the filter paper, use the plastic water spray bottle and spray the inside surface of the filter to ensure that all the blue solution has passed through the filter.
So here we have a jar containing the filtered blue liquid with all our silver dissolved in it. But how do we get the liquid to release the dissolved silver back as a solid material ? Well, it just so happens that when a piece of solid copper is placed into the blue liquid, copper atoms in the solid copper and silver atoms in the blue liquid immediately start to exchange places. The solid copper begins to slowly dissolve and a grey, foam-like material begins to appear and fall to the bottom of the glass tube.
This grey material is pure silver ! Wooohoooo !
A small copper cylinder would do the job nicely but for our purposes, I'm simply going to use an old electrical power cable like the one that plugs into a computer or a kettle, etc and strip out one of the copper wires. Then I'll wind the copper wire around a pencil to create a rough helix shape.

Take this piece of copper wire and drop it into the glass jar containing the filtered blue liquid.

Within seconds, you should see a grey, foam-like material begin to coat the wire. When it gets heavy enough, it will detach from the wire and fall to the bottom of the jar and more will start to form. Otherwise a slight shake of the jar should be sufficient to dislodge it.

If the copper wires dissolves completely, then add another similar piece of copper wire and repeat until there is no further grey material visible on the wire. This means that virtually all the silver has been removed from the blue solution and should now be at the bottom of the glass jar in the form of a grey sediment.
Now we're going to "wash" the grey material to remove all traces of remaining blue liquid.
We'll prepare another filter as we did before and fit it into the plastic funnel. Now the funnel is placed into the original glass jar that we dissolved the coins in and very slowly and carefully, the blue liquid and grey sediment is poured into the funnel. We want to let the filter catch every last bit of grey sediment.

When done, the water spray bottle will be used to spray the grey sediment in the filter until the water coming out of the bottom of the funnel is clear.
All of the liquid in the glass jar can now be tipped into a bucket, diluted with lots of water and then can be safely discarded by pouring down the sink drain ... no damage will occur.
Continued next post ...
Almost immediately, you'll see a bubbling reaction begin and the liquid start to change colour from clear to a very pretty blue shade. This blue colour is due to the copper in the coins reacting with the nitric acid to produce copper nitrate.
Also, brown vapours of nitrogen dioxide will also form.
WARNING Nitrogen dioxide is a NASTY, NASTY, NASTY gas and under no circumstances should you breathe it in. As mentioned earlier, do all this refining in the open air and you'll be perfectly ok.

To speed up the reaction, we can place the glass tube into a bath of boiling hot water. As the bath water cools, replace with fresh, boiling water.

Now, we sit back and let the reaction continue until both coins have been completely dissolved leaving behind just a brilliant blue liquid.

When the coins are completely dissolved, we need to dilute this blue liquid a bit so add an equal volume of water.
Next, we need to remove any sediment by pouring this blue liquid through a filter into the 2nd of our glass jars.
Here's how to create a suitable filter using one of the coffee filters.
1. Use a round lid to mark out a circular pattern on the filter.
2. Cut out the circular shape.
3. Fold the circular filter in half ... then fold in half again.
4. Carefully open up the filter and you'll find it should fit easily into the plastic funnel.

Place the funnel and filter into the opening of the 2nd (empty) glass jar and carefully pour the blue liquid into the funnel, allowing it to drip through.

By the way, you ARE wearing your plastic gloves, aren't you ? If you splash the blue liquid onto skin you'll get a harmless black discoloration that will take about 4 days to wear off. So wear your gloves !! That black discoloration is caused by silver in the blue liquid reacting to light and darkening ... the basis of how photographic film works :-)
Should have taken my own advice

Ok, we want to get every last bit of silver so once the liquid has finished running through the filter paper, use the plastic water spray bottle and spray the inside surface of the filter to ensure that all the blue solution has passed through the filter.
So here we have a jar containing the filtered blue liquid with all our silver dissolved in it. But how do we get the liquid to release the dissolved silver back as a solid material ? Well, it just so happens that when a piece of solid copper is placed into the blue liquid, copper atoms in the solid copper and silver atoms in the blue liquid immediately start to exchange places. The solid copper begins to slowly dissolve and a grey, foam-like material begins to appear and fall to the bottom of the glass tube.
This grey material is pure silver ! Wooohoooo !
A small copper cylinder would do the job nicely but for our purposes, I'm simply going to use an old electrical power cable like the one that plugs into a computer or a kettle, etc and strip out one of the copper wires. Then I'll wind the copper wire around a pencil to create a rough helix shape.

Take this piece of copper wire and drop it into the glass jar containing the filtered blue liquid.

Within seconds, you should see a grey, foam-like material begin to coat the wire. When it gets heavy enough, it will detach from the wire and fall to the bottom of the jar and more will start to form. Otherwise a slight shake of the jar should be sufficient to dislodge it.

If the copper wires dissolves completely, then add another similar piece of copper wire and repeat until there is no further grey material visible on the wire. This means that virtually all the silver has been removed from the blue solution and should now be at the bottom of the glass jar in the form of a grey sediment.
Now we're going to "wash" the grey material to remove all traces of remaining blue liquid.
We'll prepare another filter as we did before and fit it into the plastic funnel. Now the funnel is placed into the original glass jar that we dissolved the coins in and very slowly and carefully, the blue liquid and grey sediment is poured into the funnel. We want to let the filter catch every last bit of grey sediment.

When done, the water spray bottle will be used to spray the grey sediment in the filter until the water coming out of the bottom of the funnel is clear.
All of the liquid in the glass jar can now be tipped into a bucket, diluted with lots of water and then can be safely discarded by pouring down the sink drain ... no damage will occur.
Continued next post ...
edit on 18/2/12 by tauristercus because: (no reason given)
Continued from previous post ...
The filter is removed from the funnel, opened out carefully so it's flat ... then left until the grey material has completely dried.

This grey material is our "raw" 99.9% pure silver ... ready for the final step to change it back into that nice shiny silver metal.
Ok, we're finally at the finishing post !
Normally when melting precious metals such as silver and gold, a proper crucible made of heat resistant material is used. Such a material is designed to withstand the approximate 1000C temperature required to melt silver and gold.
However, we're going to go low tech in our approach and instead of a high temp crucible, we're going to use a potato placed ontop of a brick ... yep, you read that correctly .. we're going to use a potato upon which we're going to melt our grey powder back into silver metal ! Believe it or not, but a slice of potato can easily and for long enough, withstand the sort of temperatures we need to melt silver.

A slice of potato is all thats needed and we need to slightly carbonize (burn) the surface. The carbon helps pick up any miniscule impurities remaining in the silver and also the carbon layer helps prevent the silver from adhering to the potato.

Now, we take a small quantity of our grey powder and place it on the potato surface. We do a small quantity at a time as it takes less time to melt and once melted, we continue adding additional small quantities and re-melt. This continues until all the grey powder has been used.


Eventually all the grey powder has been melted down.

And we're left holding a "button" of 99.9% pure silver to be added with the rest of our "long term investment" portfolio of silver "buttons".

The above may have been long winded in explanation but I wanted to make sure that every step of the process was as clear as possible making it easy for anyone interested enough to have a go at themselves.
Summarized, the process is simply:
1. dissolve the scrap silver completely in nitric acid
2. filter the resulting blue solution to remove impurities
3. use a piece of copper to "drop" the silver as a grey powder
4. melt the grey powder back into metallic silver
If there's sufficient positive response, then I'll certainly consider creating a thread on how to produce 99.9% 24k gold at home ... it's almost as easy as producing silver.
The filter is removed from the funnel, opened out carefully so it's flat ... then left until the grey material has completely dried.

This grey material is our "raw" 99.9% pure silver ... ready for the final step to change it back into that nice shiny silver metal.
Ok, we're finally at the finishing post !
Normally when melting precious metals such as silver and gold, a proper crucible made of heat resistant material is used. Such a material is designed to withstand the approximate 1000C temperature required to melt silver and gold.
However, we're going to go low tech in our approach and instead of a high temp crucible, we're going to use a potato placed ontop of a brick ... yep, you read that correctly .. we're going to use a potato upon which we're going to melt our grey powder back into silver metal ! Believe it or not, but a slice of potato can easily and for long enough, withstand the sort of temperatures we need to melt silver.

A slice of potato is all thats needed and we need to slightly carbonize (burn) the surface. The carbon helps pick up any miniscule impurities remaining in the silver and also the carbon layer helps prevent the silver from adhering to the potato.

Now, we take a small quantity of our grey powder and place it on the potato surface. We do a small quantity at a time as it takes less time to melt and once melted, we continue adding additional small quantities and re-melt. This continues until all the grey powder has been used.


Eventually all the grey powder has been melted down.

And we're left holding a "button" of 99.9% pure silver to be added with the rest of our "long term investment" portfolio of silver "buttons".

The above may have been long winded in explanation but I wanted to make sure that every step of the process was as clear as possible making it easy for anyone interested enough to have a go at themselves.
Summarized, the process is simply:
1. dissolve the scrap silver completely in nitric acid
2. filter the resulting blue solution to remove impurities
3. use a piece of copper to "drop" the silver as a grey powder
4. melt the grey powder back into metallic silver
If there's sufficient positive response, then I'll certainly consider creating a thread on how to produce 99.9% 24k gold at home ... it's almost as easy as producing silver.
edit on 18/2/12 by tauristercus because: (no reason given)
:bowdown:
I`ll probably never use this knowledge, but thankyou for taking the time, I enjoyed reading all that for some reason lol super interesting.
I`ll probably never use this knowledge, but thankyou for taking the time, I enjoyed reading all that for some reason lol super interesting.
Fantastic thread ,I didnt know it was that easy in fact im going to try this for myself,if you dont mind me asking what did the bag of old coins cost
Great money making idea for yourself, is that a blowtorch your using for the fire?
edit on 18-2-2012 by anthonygillespie2012 because: (no
reason given)
reply to post by tauristercus
A very cool thread OP! I'm more a silver lover than a gold lover too, I only have 2 pieces of jewellery and they are both silver.
1 question. I know that the price of silver is usually more stable and constant than that of gold but how much did the coins cost you compared to the value of the silver contained within them? Would it be possible for more of us to use that as a way of supplementing our income?
A very cool thread OP! I'm more a silver lover than a gold lover too, I only have 2 pieces of jewellery and they are both silver.
1 question. I know that the price of silver is usually more stable and constant than that of gold but how much did the coins cost you compared to the value of the silver contained within them? Would it be possible for more of us to use that as a way of supplementing our income?
reply to post by Bilder
I paid approximately $AUS 20 for the half kilo bag.
It certainly pays to keep an eye out on eBay as quite often really good bargains show up which are worth snapping up.
I paid approximately $AUS 20 for the half kilo bag.
It certainly pays to keep an eye out on eBay as quite often really good bargains show up which are worth snapping up.
Awsome Demo!! I have about 45gm of 14k jewelry gold that I melted down and want to refine it. Can this same method be used with gold? What other
metals are mixed in 14k gold? (copper, silver, nickel)? Also, what do you do with the remaining Nitric Acid solution with copper in it? are you able
to refine the copper from the solution?
I just looked up the cost of nitric acid here in San Diego 1L cost around $30 - 45...How much Nitric acid would be used to disolve those 2 coins?
I just looked up the cost of nitric acid here in San Diego 1L cost around $30 - 45...How much Nitric acid would be used to disolve those 2 coins?
edit on 19-2-2012 by Alchemst7 because: (no reason given)
Originally posted by anthonygillespie2012
Great money making idea for yourself, is that a blowtorch your using for the fire?edit on 18-2-2012 by anthonygillespie2012 because: (no reason given)
It's a propane torch available at almost every hardware store.
It easily generates a flame capable of very quickly melting silver and gold.
In the tutorial, it took approximately 60 secs in total to convert all that grey powder back into metallic silver.
Originally posted by LightSpeedDriver
reply to post by tauristercus
A very cool thread OP! I'm more a silver lover than a gold lover too, I only have 2 pieces of jewellery and they are both silver.
Would it be possible for more of us to use that as a way of supplementing our income?
I suppose it would. I'm certainly looking at the long term silver market and expecting to see quite reasonable increases in silver value over the next say 2, 5 or 10 years. Silver is NOT a renewable resource, what's in the ground is all there ever will be and considering that silver is sought after in huge quantities by the electronic industries and as jewelry, it stands to reason that there'll be less and less of it available over time thereby driving the price up.
reply to post by tauristercus
I would like to try this myself just for the fun thanks for the info, are there any dangers involved making them? I have never created anything like this before but really want to give it a try as long as nothing poisonous kills me or something blows up. I noticed your wearing gloves.
I would like to try this myself just for the fun thanks for the info, are there any dangers involved making them? I have never created anything like this before but really want to give it a try as long as nothing poisonous kills me or something blows up. I noticed your wearing gloves.
edit
on 19-2-2012 by anthonygillespie2012 because: (no reason given)
Really interesting thread!
A few questions though.
How much does that blob of silver in your hand weigh at the end of the process?
How much did the chemicals and materials cost so you could do this i.e. the acid, gas, filters, gloves ?
How long did the process take you from start to finish? including the time buying the coins on ebay, prepping etc
A few questions though.
How much does that blob of silver in your hand weigh at the end of the process?
How much did the chemicals and materials cost so you could do this i.e. the acid, gas, filters, gloves ?
How long did the process take you from start to finish? including the time buying the coins on ebay, prepping etc
That is cool. I have been interested in doing this for some time. Now I know where to start. I will be looking forward to a thread about gold as well.
Thanks
That's impressive.. I certainly didn't think you could do that.. Thanks.. BTW, when heating the powder at the end, can you use something other than
that blow torch thing? Don't think I can get hold of one of them so easily, lol..
I love your choice of forum to post this in.
Please do continue on with the gold process too.
I was thinking you could do every part of this process except the last and store it in a urn. Nobody would ever know to steal/confiscate it.
Please do continue on with the gold process too.
I was thinking you could do every part of this process except the last and store it in a urn. Nobody would ever know to steal/confiscate it.
Originally posted by Alchemst7
Awsome Demo!! I have about 45gm of 14k jewelry gold that I melted down and want to refine it. Can this same method be used with gold? What other metals are mixed in 14k gold? (copper, silver, nickel)? Also, what do you do with the remaining Nitric Acid solution with copper in it? are you able to refine the copper from the solution?
Because gold is a relatively soft metal, it's usually mixed with quantities of silver and other various common metals such as copper to give it more of a hardness.
24 carat gold is almost pure gold
18 carat gold is approximately 75% gold and 25% other less valuable metals
9 carat gold is approximately 37% gold and 63% other less valuable metals.
That 14gms of 14k gold contains approximately 58% gold which works out to approximately 8gms of 24k gold and 6 gms of other metals.
At current market gold rates, that 8ms of gold when extracted should be worth around $US 440.
reply to post by anthonygillespie2012
Nitric acid is used in the process. This is something that a great deal of respect and attention must be shown when handling. It will strip paint from a door, exude nasty fumes in the process and possibly give you some serious explaining to do when a significant other comes home.
reply to post by tauristercus
Perhaps a more practical question. Don't people look at you funny when you come in "off the street" with silver purer than is generally used in coin-making or jewellery? "Are ya making this at home there are ya Joe?"
Nitric acid is used in the process. This is something that a great deal of respect and attention must be shown when handling. It will strip paint from a door, exude nasty fumes in the process and possibly give you some serious explaining to do when a significant other comes home.
reply to post by tauristercus
Perhaps a more practical question. Don't people look at you funny when you come in "off the street" with silver purer than is generally used in coin-making or jewellery? "Are ya making this at home there are ya Joe?"
edit on 19/2/12 by LightSpeedDriver because: Added a
reply
new topics
-
America's Greatest Ally
General Chit Chat: 14 minutes ago -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 5 hours ago -
Maestro Benedetto
Literature: 6 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 6 hours ago -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 10 hours ago -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 11 hours ago -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 11 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 5 hours ago, 26 flags -
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest: 16 hours ago, 9 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 11 hours ago, 8 flags -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics: 16 hours ago, 8 flags -
Weinstein's conviction overturned
Mainstream News: 14 hours ago, 8 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 13 hours ago, 7 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 10 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 13 hours ago, 5 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 11 hours ago, 4 flags -
Is AI Better Than the Hollywood Elite?
Movies: 6 hours ago, 3 flags
active topics
-
Is AI Better Than the Hollywood Elite?
Movies • 16 • : 5thHead -
Hate makes for strange bedfellows
US Political Madness • 48 • : Terpene -
America's Greatest Ally
General Chit Chat • 0 • : 19Bones79 -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 17 • : BingoMcGoof -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 29 • : 19Bones79 -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics • 90 • : Lumenari -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs • 12 • : KrustyKrab -
SHORT STORY WRITERS CONTEST -- April 2024 -- TIME -- TIME2024
Short Stories • 23 • : DontTreadOnMe -
Truth Social goes public, be careful not to lose your money
Mainstream News • 130 • : Astyanax -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 689 • : daskakik
