It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: oriondc
There are more methods than radiocarbon dating used, especially when examining geological strata, ejecta, etc. Uranium series, fission track, luminescence, cosmogenic nuclides, magnetostratigraphy... many methods. By determining the geological material age, whatever is found in it can also be dated.
Exactly!
Scientists use numerous dating methods to come up with an approximate date. The following link describes in detail these dating methods, and how they are applied for those still struggling.
www.nature.com...
originally posted by: TheConstruKctionofLight
a reply to: camain
Cool. So the biggest conspiracy of all time is that we don't have an accurate method of dating anything older than 50,000 years.
Where do they get these wild figures?
"Such and such happened 50 mill years ago, then something else happened 2 million years ago?"
There are multiple ways to date fossils:
www.abovetopsecret.com...
originally posted by: Phantom423
There are multiple ways to date fossils:
www.abovetopsecret.com...
There are no reliable ways to date radiometrically. The problem is that we never know the original isotopic ratio. Without the original isotopic ratio ("initial amount" in the equation below) you cannot solve for time in the half-life equation.
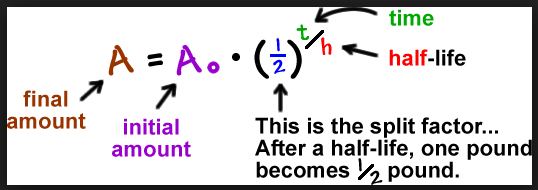
Its as simple as that. They can conjecture all they want, and most people will eat it up because they believe anything the theorists say, but the fact of the matter is they are speculating. Isochron dating also does not give an unambiguous initial concentration.
The closest we have to accurate radiometric dating is carbon dating. We can assume to some degree that the isotopic ratio of C-14 to regular carbon has remained mostly consistent in the atmosphere throughout time. Yet we do not know for sure, so even carbon dating, which would have the least variability, still has an immense possibility for error.
edit on 26-9-2020 by cooperton because: (no reason given)
This actually kind of cool but also kind of scary.
The fact that microbes come back after 60 days is hopeful that life will survive in the case of our planet being ripped apart.
On the other hand, our garbage is doing the same.
As far as dating goes... we know the rate of sedimentation. The dates have been carbon dated from various spots around the world. Unless there is a magic process that can deposit more in one spot (re: C14), then it is assumed that it equivalent around the world (this is done to “six sigma” or a millionth standard deviation. It has rechecked, verified, and usually repeated with even more scrutiny). I think it is valid methodology and valid interpretation (and extrapolation) of natural processes.
Extremeophiles, amino acids on asteroids, us, cockroaches, tardigrades, and Cher,... life Just IS.
And if QM is to be believed, the existence of Universe is manifest upon the fact that life is everywhere!!
What we should be asking is, “is it wise to revive such ancient life”?? (Erm, coronavirus??!)
Yet more melancholy from TEOT!!
The fact that microbes come back after 60 days is hopeful that life will survive in the case of our planet being ripped apart.
On the other hand, our garbage is doing the same.
As far as dating goes... we know the rate of sedimentation. The dates have been carbon dated from various spots around the world. Unless there is a magic process that can deposit more in one spot (re: C14), then it is assumed that it equivalent around the world (this is done to “six sigma” or a millionth standard deviation. It has rechecked, verified, and usually repeated with even more scrutiny). I think it is valid methodology and valid interpretation (and extrapolation) of natural processes.
Extremeophiles, amino acids on asteroids, us, cockroaches, tardigrades, and Cher,... life Just IS.
And if QM is to be believed, the existence of Universe is manifest upon the fact that life is everywhere!!
What we should be asking is, “is it wise to revive such ancient life”?? (Erm, coronavirus??!)
Yet more melancholy from TEOT!!
originally posted by: cooperton
originally posted by: Phantom423
There are multiple ways to date fossils:
www.abovetopsecret.com...
There are no reliable ways to date radiometrically. The problem is that we never know the original isotopic ratio. Without the original isotopic ratio ("initial amount" in the equation below) you cannot solve for time in the half-life equation.
Its as simple as that. They can conjecture all they want, and most people will eat it up because they believe anything the theorists say, but the fact of the matter is they are speculating. Isochron dating also does not give an unambiguous initial concentration.
The closest we have to accurate radiometric dating is carbon dating. We can assume to some degree that the isotopic ratio of C-14 to regular carbon has remained mostly consistent in the atmosphere throughout time. Yet we do not know for sure, so even carbon dating, which would have the least variability, still has an immense possibility for error.
Strange that hundreds of scientists use radiometric dating for all sorts of things including medical diagnostics. Do you tell your doctor that he/she hasn't a clue about radiometric techniques? Creationists are the only idiots who refuse to acknowledge modern science. Same old, same old. Carry on.
Wait - I have a better idea! Why don't you write the authors a letter! Authors: Darryl E. Granger1
, Ryan J. Gibbon2
, Kathleen Kuman3,4, Ronald J. Clarke3
, Laurent Bruxelles4,5,6 & Marc W. Caffee1,7
Here's part of the abstract describing the radiometric technique. I'm sure they'll be very interested to know that they wasted their time!
New cosmogenic burial ages for Sterkfontein
Member 2 Australopithecus and Member 5 Oldowan
Burial dating is based on the radioactive decay of 26Al and 10Be in
quartz. These nuclides build up by exposure to secondary cosmic radiation near the ground surface, and subsequently decay when sediment
is buried and cosmogenic nuclide production is attenuated. Because
26Al (t26 5 1.021 6 0.024 Myr (ref. 28)) decays faster than 10Be
(t10 5 2.005 6 0.020 Myr (ref. 29)), the ratio 26Al/10Be decreases over
time, with an effective mean-life of tbur 5 2.086 0.10 Myr. For burial
dating to be accurate, three criteria must be met. (1) The quartz must be
exposed near the ground surface before burial to accumulate sufficient
26Al and 10Be. (2) It must be buried quickly and deeply enough so that
post-burial production is small. The exact depth required depends
upon the inherited concentrations, but is usually many metres. (3) It
must be buried only once in the past ,10 Myr. If quartz has been
reworked from older deposits, or if it has been reworked underground
within the cave system, then the burial age will overestimate the true
age of the deposit.
An elegant way to test whether the burial dating criteria are met is to
construct an isochron24–27 in which multiple samples are analysed from
the same location. Each sample is buriedwith its own inherited 26Al and 10Be concentrations, but all samples share the same post-burial production history. A plot of 26Al versus 10Be yields a gentle curve with a slope
that indicates burial age and an intercept that depends on the amount of
post-burial production24. The isochron burial dating method accounts
for post-burial production without requiring detailed knowledge of the burial depth or burial history. It also allows outliers to be identified;
reworked samples plot below the isochron, while samples significantly
above the isochron are forbidden and indicate issues with either the
sample or the laboratory measurements.
We analysed 11 samples from Member 2 (Table 1), including three
previously reported
. Effective isochron burial dating requires a wide
range of inherited cosmogenic nuclide concentrations. To that end, we
selected a suite of samples to maximize variability. Fine quartz sandfrom
multiple samples (ST 1–9) was probably washed in from the surface. In
contrast, four blocks of chert were collected from the immediate vicinity
of StW 573 (M2CA–D). Two fractions of coarse sand and pebbles were
separated (ST M2 Dark and Light). One fraction comprises rounded
grains stained with pedogenic iron oxides and washed into the cave from
soil at the surface; the second comprises angular unstained grains probably eroded from the walls and ceiling of the cave itself (Extended Data
Fig. 1). A previously reported sample from the modern surface9 was
analysed to confirm that material enters the cave with a zero burial age
Holy cow!! Here's another paper! You're going to be busy writing those letters. Better start asap!
www.scienceandculture-isna.org...
Conclusions
The characteristic nuclear property of radioactive
decay of radionuclides at almost immutable rates has been
effectively utilized to develop over forty different
radiometric techniques for dating of varieties of materials
of geological and biological origins since its initiation about
100 years back. With gradual improvement in nuclear
techniques, in general,the methods of absolute dating as
analytical tools have undergone continual refinement
resulting in enhancement in accurate and precise dating of
important geological events as well as the history of
organisms once living on earth.
Application of efficient radiometric dating enables the
geologists to record the time sequence of different
geological formations involving the age of the earth,
meteorites, lunar rocks, etc., within the geologic time scales
and also encourages the archaeologists to study ancients
events of biological origins such as dinosaur era, age of
the Iceman, the Shroud in Turin and also in authentication
of important archaeological materials along with
their evolutionary changes. In dating, absolute age is just a
fancy way of presenting the definitive or specific age of a
material against the relative age which only refers to how
old or young a substance is in comparison to something
else. Close agreement in dating of the age of the earth
derived through different dating methods, for example, is
a hallmark of nuclear or radiometric dating. In fact, the
radioactive decay constitutes a ‘clock’ provided by
nature.
And another holy cow! They actually teach this stuff in schools!!!
TEACHING THE MATHEMATICS OF RADIOMETRIC DATING
James H. Shea Geology Department, University of Wisonsin - Parkside, Box number 2000, Kenosha, Wl 53141,
[email protected]
www.tandfonline.com...
You better advise Professor Shea that he should retract his book. In fact, why don't you sue him for distributing false information? That would get some attention!
edit on 27-9-2020 by Phantom423 because: (no reason given)
a reply to: cooperton
Another holy cow! They even teach radiometric dating in schools!!! You better get on your horse and advise Professor Shea to retract his book immediately. You might consider suing him for distributing false information. That would get some attention!
Teaching the Mathematics of Radiometric Dating
James H. Shea
Pages 22-24 | Published online: 31 Jan 2018
Download citation doi.org...
www.tandfonline.com...
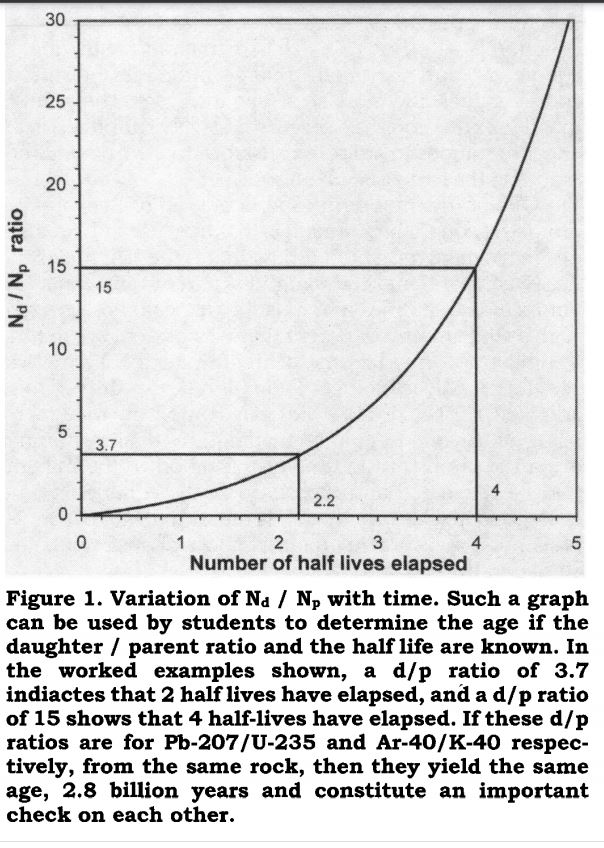
Another holy cow! They even teach radiometric dating in schools!!! You better get on your horse and advise Professor Shea to retract his book immediately. You might consider suing him for distributing false information. That would get some attention!
Teaching the Mathematics of Radiometric Dating
James H. Shea
Pages 22-24 | Published online: 31 Jan 2018
Download citation doi.org...
www.tandfonline.com...

originally posted by: Phantom423
In this chart they are assuming they know the initial concentration. In reality/in the field you cannot know the initial concentration of a sample. This makes discerning the age of the sample impossible.
Half-life is a highly precise science, but if you don't know the initial concentration, then you can't determine the age of the sample (t).
a reply to: cooperton
Write them all a letter with your advanced mathematics. You're the perfect example of willful ignorance. The calculation as well as the rationale for initial concentrations is in every geology textbook but you still insist that you're right and everyone else is wrong.
www.nps.gov...
Write them all a letter with your advanced mathematics. You're the perfect example of willful ignorance. The calculation as well as the rationale for initial concentrations is in every geology textbook but you still insist that you're right and everyone else is wrong.
To determine the ages in years of Earth materials and the timing of geologic events such as exhumation and subduction, geologists utilize the process of radiometric decay. Geologists use these dates to further define the boundaries of the geologic periods shown on the geologic time scale. Radiometric decay occurs when the nucleus of a radioactive atom spontaneously transforms into an atomic nucleus of a different, more stable isotope. This transformation happens via the emission of particles such as electrons (known as beta decay) and alpha particles. For instance, rubidium-87 (87Rb), an unstable element, becomes strontium-87 (87Sr), a stable element, via beta decay. As explained on WebGeology from the University of Tormsø, Norway: One neutron of the nucleus emits a beta particle, which is identical to an electron. In addition the neutron emits a neutral particle that is called an antineutrino. By emitting a beta particle, the neutron is transformed into a proton. This results in a nucleus composed of 38 protons and 49 neutrons, corresponding to strontium’s nucleus of 87 atomic particles. Energy is released during this process. The rubidium-strontium method has been a popular method to determine the absolute age of geological processes.
When discussing decay rates, scientists refer to “half-lives”—the length of time it takes for one-half of the original atom of the radioactive isotope to decay into an atom of a new isotope.Because decay occurs at a fixed rate (this is the key point), scientists can measure the amount of decayed material in the sample, determine the ratio between original and decayed material, and then calculate the sample’s age. Depending on the half-life and the material being dated, various methods are used. For instance, geologists use the Sm-Nd (samarium-147/neodymium-143) method for determining the age of very old materials (e.g., meteorites and metamorphic rocks) or when a rock became crystallized (in the mantle) or metamorphosed (at a subduction zone). For young organic materials, the carbon-14 (radiocarbon) method is used. The effective dating range of the carbon-14 method is between 100 and 50,000 years.
www.nps.gov...
a reply to: Phantom423
None of that says how they could accurately determine the initial isotopic ratio. I know how the equation works its very simple algebra. You need to know the initial concentration if you want to determine the age of the sample.
None of that says how they could accurately determine the initial isotopic ratio. I know how the equation works its very simple algebra. You need to know the initial concentration if you want to determine the age of the sample.
a reply to: cooperton
No. That is the point!
You extrapolate back from known conditions.
Yeah, anyone can question it but the question would be in question at that point (usually political).
Science doesn’t care about being wrong. It actually invites it! But it too has to be scientific as well.
All else is opinion.
PS - The data seems to show that their own ideas are wrong even with an eloquent argument the other way. My 2☯️ (sense! lol)
No. That is the point!
You extrapolate back from known conditions.
Yeah, anyone can question it but the question would be in question at that point (usually political).
Science doesn’t care about being wrong. It actually invites it! But it too has to be scientific as well.
All else is opinion.
PS - The data seems to show that their own ideas are wrong even with an eloquent argument the other way. My 2☯️ (sense! lol)
originally posted by: TEOTWAWKIAIFF
a reply to: cooperton
No. That is the point!
You extrapolate back from known conditions.

If you want to determine age of the sample (t) you need to know the initial concentration. To extrapolate and speculate regarding the initial concentration is not good scientific practice. It seems too crazy to be true... that all those massive million year old dates that we've been told regarding things are just total speculation. But it is - complete speculation. Don't try beating around the bush, it is as simple as that. If you don't know with certainty what the initial concentration is, you cannot know how old the sample is!
edit on 27-9-2020 by cooperton because:
(no reason given)
a reply to: FinallyAwake
BullSh#t! I flat out refuse to believe science anymore.
It's filled with Bullsh#t.
BullSh#t! I flat out refuse to believe science anymore.
It's filled with Bullsh#t.
edit on 28-9-2020 by carsforkids because: (no reason given)
originally posted by: carsforkids
a reply to: FinallyAwake
BullSh#t! I flat out refuse to believe science anymore.
It's filled with Bullsh#t.
The problem is we can't fully trust the interpretation given by scientists anymore. We have to look at the data ourselves. If someone is incapable of looking at the data for their selves, then they will likely just blindly believe what the white coats say. When I researched into Radiometric dating, that really did it for me. I was astonished that it's a total guessing game. I've read some papers where they assume the initial concentration of the radioactive isotope is 100%, which is crazy, because a 100% pure sample is literally never found in nature. They simply made up the initial concentration that would best fit their narrative.
originally posted by: TEOTWAWKIAIFF
a reply to: cooperton
It is not a single sample. And it is not only local.
So, global, and delta over time... a different argument.
Doesn't matter how many samples you have, you can never know what the initial concentration of a radiometric sample is. Closest we can get is carbon-dating, but even within that there is so much potential variability.
edit on 28-9-2020 by cooperton because: (no reason given)
originally posted by: carsforkids
a reply to: FinallyAwake
BullSh#t! I flat out refuse to believe science anymore.
It's filled with Bullsh#t.
Then why are you using the internet??????? You should be working on your stone tools and paintings on cave walls.
originally posted by: Phantom423
Then why are you using the internet??????? You should be working on your stone tools and paintings on cave walls.
By context you should know he's talking about the theoretical scientists who lead multitudes into delusion. He's referring to people like you who bully anyone with a dissenting opinion. You resort to insult rather than cordial discussion when your beliefs are threatened. He no longer believes in your scientism religion. Real science remains pure.
Which brings me back to my point - you can't determine the age of a radioactive sample if you don't know the initial concentration of the isotope. You refuse to accept this because you belong to the church of scientism, and you actually hate science.
originally posted by: cooperton
originally posted by: Phantom423
Then why are you using the internet??????? You should be working on your stone tools and paintings on cave walls.
By context you should know he's talking about the theoretical scientists who lead multitudes into delusion. He's referring to people like you who bully anyone with a dissenting opinion. You resort to insult rather than cordial discussion when your beliefs are threatened. He no longer believes in your scientism religion. Real science remains pure.
Which brings me back to my point - you can't determine the age of a radioactive sample if you don't know the initial concentration of the isotope. You refuse to accept this because you belong to the church of scientism, and you actually hate science.
Once again, write them a letter. Let's see what they have to say about your "opinion". Don't forget to include your latest mathematical tricks. What are you afraid of? Being laughed off the internet?
edit on 28-9-2020 by Phantom423 because: (no reason given)
new topics
-
CIA is alleged to be operat social media troll frms in Kyiv
ATS Skunk Works: 19 minutes ago -
Rainbow : Stargazer
Music: 56 minutes ago -
I sleep no more.
Philosophy and Metaphysics: 3 hours ago -
Canada caught red-handed manipulating live weather data and make it warmer
Fragile Earth: 3 hours ago -
Why Files Our Alien Overlords | How We Secretly Serve The Tall Whites
Aliens and UFOs: 4 hours ago -
Curse of King Tuts Tomb Solved
Ancient & Lost Civilizations: 6 hours ago -
What allies does Trump have in the world?
ATS Skunk Works: 6 hours ago
top topics
-
BIDEN Admin Begins Planning For January 2025 Transition to a New President - Today is 4.26.2024.
2024 Elections: 15 hours ago, 10 flags -
Canada caught red-handed manipulating live weather data and make it warmer
Fragile Earth: 3 hours ago, 10 flags -
Big Storms
Fragile Earth: 16 hours ago, 9 flags -
Why Files Our Alien Overlords | How We Secretly Serve The Tall Whites
Aliens and UFOs: 4 hours ago, 8 flags -
Curse of King Tuts Tomb Solved
Ancient & Lost Civilizations: 6 hours ago, 6 flags -
What allies does Trump have in the world?
ATS Skunk Works: 6 hours ago, 3 flags -
I sleep no more.
Philosophy and Metaphysics: 3 hours ago, 1 flags -
CIA is alleged to be operat social media troll frms in Kyiv
ATS Skunk Works: 19 minutes ago, 1 flags -
Rainbow : Stargazer
Music: 56 minutes ago, 0 flags
active topics
-
So this is what Hamas considers 'freedom fighting' ...
War On Terrorism • 275 • : YourFaceAgain -
CIA is alleged to be operat social media troll frms in Kyiv
ATS Skunk Works • 1 • : FlyersFan -
Big Storms
Fragile Earth • 18 • : bluesman023 -
Curse of King Tuts Tomb Solved
Ancient & Lost Civilizations • 5 • : diaclonethunder -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 344 • : DBCowboy -
BIDEN Admin Begins Planning For January 2025 Transition to a New President - Today is 4.26.2024.
2024 Elections • 26 • : bluesman023 -
Canada caught red-handed manipulating live weather data and make it warmer
Fragile Earth • 12 • : YourFaceAgain -
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest • 22 • : FlyersFan -
I sleep no more.
Philosophy and Metaphysics • 6 • : AcrobaticDreams1 -
Shocking Number of Voters are Open to Committing Election Fraud
US Political Madness • 8 • : Kaiju666
