It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Mary Rose
Not true.
If you see it, it has shape, and can be drawn with a picture or a word picture.
I read this, and don't know whether to laugh or facepalm.
About fifty things crossed my mind to plop down here and say "Ok, then, look at this picture and tell me..." from statics to dynamics, to basic circuits, to antenna theory, to strengths of materials, to chemistry, to thermo.
Not a one of which you can look at a picture and 'know' the answer.
It's not worth the effort though. I'm not sure you'd even get the point.
Bedlam
Not a one of which you can look at a picture and 'know' the answer.
Oh you have to have words to go along with the picture, of course!
Mary Rose
Bedlam
Thus, you determine what makes a difference and what does not, and eliminate the unnecessary bits.
Again, going back to five systems listed for thermodynamics: Only one exists, in reality, according to you?
Bedlam
Some things you can't see without calculations.
Not true.
If you see it, it has shape, and can be drawn with a picture or a word picture.
The five systems are merely a representation of what can be gotten out of said thermodynamic system. In reality, we live in a giant closed (or open, depending on your view of the universe, its birth, its end, etc.) The reason you can say the universe is a closed system without actually knowing what lays beyond it, is by the empirical data collected for a very long time since we discovered how to collect it.
Legend:
+ (energy)
= (border of the system where no energy potentials can pass)
- (open border of the system that energy or potential energy can come from)
A closed system looks like this:
= ++++++ =
An open system looks like this:
= ++++++ --- +++++++++++++++++++++++++++++++
A battery is a closed system. It has only so much energy potential inside of it. When its energy is expended, it's goodnight battery. Plug it into a charger and voila, you now have an open system. The battery and the charger, and the outlet which powers the charger, which leads back to the power plant burning coal which in turn powers your battery which in turn powers your iPod.
Simple as that really.
I am trying to be as basic as I can here so you get a firm grasp on thermodynamics.
To increase our understanding a little more, you can look at a gasoline engine. The thermodynamic system includes not only the gas you put into the car, but the heat that is dissipated when the engine burns fuel which comes from the energy potentials originally found in the gas.
If we have a litre of gas, which has about 42MJ of energy. We know that burning it that 42MJ will go somewhere. If gasoline engines run at 30% efficiency, we know that 12MJ will be converted to mechanical energy.
The most important thing that thermodynamics tells us, is that only 42MJ can be produced, and they can be accounted for along the way!
12 MJ made it to actually pushing the car around. But the other 70% was lost energy due to heat created in the engine, wind resistance, friction, braking, etc.
All of this is considered a "load". Now, Bedini et al, all like to 'invent' these great free energy machines, yet all of them are very shy at adding load (resistance) to them. If you have a system that creates unlimited energy, "tapping into it" (a very popular term in FE circles) requires adding some kind of load (resistance) to it.
That can be just about anything requiring energy to operate, and we are in no shortage of supply for such items!
***
It is no wonder that people who freely take in any gobbledegook about free energy usually have no concept of thermodynamic systems.
I really don't mean that in a negative way against you, merely it is aimed at the people trying to take advantage of your ignorance. (And I don't mean ignorance in a bad way either, we all have areas of whatever in our lives that we are good at. Some very talented, smart, famous people have been suckered into bad investments sitting down in closed investor groups where they understand nothing that is going on. (See big words, think big dreams.)
Your earlier diagram:
Type of system Mass flow - Work - Heat
Is simply describing different types of thermodynamic systems. If we take the above examples we can apply it to the chart:
irst Law A statement of the conservation of energy in a thermodynamic system. Net energy crossing a system boundary equals the energy change inside the system.
Heat (Q) is energy transferred due to a temperature difference and is considered positive if it is inward or added to the system.
An open system allows energy to cross the boundaries we spoke about in the above examples. A closed system, doesn't. (The outside boundaries anyway).
The chart is speaking about interactions, so I assume it is laying out the type of interactions possible in said systems.
An open system, can do:
Mass flow (aka: rushing water/gasoline [matter] into the system)
Work: Mechanical energy (load)
Heat: Heat
n a closed system, no mass may be transferred in or out of the system boundaries. The system always contains the same amount of matter, but heat and work can be exchanged across the boundary of the system. Whether a system can exchange heat, work, or both is dependent on the property of its boundary.
en.wikipedia.org...
The very definition of a closed system does not allow things to cross the system boundaries. An open system allows whatever to cross the boundaries. I believe you made an Atmos clock thread. That is an example of an open system. Using energy from the environment to power itself. A more accurate example of a completely open system would be a dam, using energy from water to power mechanical energy. All derived from the sun. You could describe hydroelectric power, as a system that starts with the water reservoir and goes down to the dam, and then the energy distributed. And you could account for all the energy along the way. You could also incorporate the sun into the system, then making it a closed system as the sun only has so much fuel (energy) in it. and one day it will cease to exist. That is why thermo systems are really about interpretation and are a construct of math.
A thermally isolated system does not allow heat transfer. If something is insulated from heat (temperature variations), there will be no transfer of energy in or out of the system from heat! (Hard to think of an example for this one. Think of a superconductor in a perfect vacuum, but the borders lying within the superconductor)
A mechanically isolated system allows no mechanical energy transfer. A simple example would be a pot of warm water with a lid on it. No matter will transfer because of the lid, but you can warm your hands on it and get heat energy from it.
And a fully isolated system can't do squat. Think of a piece of non-themally conductive piece of styrofoam. Just sitting there waiting for some kid to mistake it for popcorn. It does absolutely squat. No energy transfer, no work, no mass going inside or outside its squishy boundaries. (sorry this one is hard to drum up).
edit on 19-11-2013 by boncho because: (no reason given)
edit on 19-11-2013 by boncho because: (no reason
given)
edit on 19-11-2013 by boncho because: (no reason given)
edit on 19-11-2013 by boncho because: (no reason
given)
Bedlam
reply to post by boncho
I actually spent an hour or so the other night trying to find a math-free yet technically correct website with lots of pictures and diagrams, maybe some animations or videos. I never found one.
There are a LOT of basic thermo websites discussing open and closed systems and heat engines and heat pumps, and why Carnot's limits exist, and how you can prove it, but none of them are going to be accessible to her because of the math. The really good ones are going to go off into DE and calculus, at best it's college algebra.
Algebra, what is that, like word pictures?
Mary Rose
Bedlam
Thus, you determine what makes a difference and what does not, and eliminate the unnecessary bits.
Again, going back to five systems listed for thermodynamics: Only one exists, in reality, according to you?
Mary, I made an attempt at explaining the interactions of the five thermodynamic systems. I hope you read them.
Bedlam
Some things you can't see without calculations.
reply to post by Mary Rose
If you see it, it has shape, and can be drawn with a picture or a word picture.
There are so many things wrong with this statement on a fundamental level. Again, I'm implore you to make an effort at reading people's replies. Many here have spent time to help you understand. If something is over your head it is better to simply admit it and work with your understanding, rather than tell people they are wrong in their fundamental understanding of things (while yours is clearly not 'outside the box' but outside the cardboard of said box.)
edit on 19-11-2013 by boncho because: (no reason given)
edit on 19-11-2013 by boncho because: (no reason
given)
edit on 19-11-2013 by boncho because: (no reason given)
Mary, to demonstrate how arbitrary a thermodynamic system is, I have created a graphic which shows once you change the parameters of the system, it
changes. Arbitrary in this case is not a bad thing, it allows us to visualize many different systems and also determine where they are open or closed,
meaning we are able to see exactly what we should be able to get out of them.
The history of "free energy" is kind of bred out of the perpetual motion machine, which aimed to violate these systems by declaring that in a closed system, we can have unlimited energy. Which is simply not true. Same goes for free energy, there is no such thing. Some people try to argue the term is related to monetary implications but that is bollocks as well because everything has cost associated with it. True free energy in the monetary sense would be aliens coming down giving us free working fusion reactors that needed no fuel replacement, maintenance, repair, distribution systems, etc.

Now, in the graphic one could argue that adding nebula to the system would turn it back into an open system, yet, you could also argue that the planet would be destroyed by then, so it's semantics.
And the universe, according to free energy proponents is powered by unicorn farts so I thought I'd add that in there.
The bottom line is: Thermodynamics tells us that every bit of energy can be calculated and accounted for. No if ands or buts.
If someone did find something to violate thermodynamics (which thousands upon thousands have tried for many a year) they would open up a whole new realm of science. It would not mean that thermodynamics is wrong, (no more than Newton was wrong) only that our understanding is limited and we need new science (see: massive funding efforts and frantic eggheads looking for a new theory) to explain this inconsistency.
So far, no one has adequately proved thermodynamics to be incorrect. Even though some claim it. The simple fact is that even if you did this by making some useless machine that couldn't even be a practical "free energy" machine, the simple fame and prestige alone (not to mention the nobel prize) would be worth far more money and status than selling DVDs, and trying to wrangle up idiot investors.
Whether or not some free energy schmuck puts "Open source" on their blog, doesn't mean they are altruistic. In fact, go through the history of these people and see they have been trying to make money the entire time. (And have been doing so shadily too.)
The history of "free energy" is kind of bred out of the perpetual motion machine, which aimed to violate these systems by declaring that in a closed system, we can have unlimited energy. Which is simply not true. Same goes for free energy, there is no such thing. Some people try to argue the term is related to monetary implications but that is bollocks as well because everything has cost associated with it. True free energy in the monetary sense would be aliens coming down giving us free working fusion reactors that needed no fuel replacement, maintenance, repair, distribution systems, etc.

Now, in the graphic one could argue that adding nebula to the system would turn it back into an open system, yet, you could also argue that the planet would be destroyed by then, so it's semantics.
And the universe, according to free energy proponents is powered by unicorn farts so I thought I'd add that in there.
The bottom line is: Thermodynamics tells us that every bit of energy can be calculated and accounted for. No if ands or buts.
If someone did find something to violate thermodynamics (which thousands upon thousands have tried for many a year) they would open up a whole new realm of science. It would not mean that thermodynamics is wrong, (no more than Newton was wrong) only that our understanding is limited and we need new science (see: massive funding efforts and frantic eggheads looking for a new theory) to explain this inconsistency.
So far, no one has adequately proved thermodynamics to be incorrect. Even though some claim it. The simple fact is that even if you did this by making some useless machine that couldn't even be a practical "free energy" machine, the simple fame and prestige alone (not to mention the nobel prize) would be worth far more money and status than selling DVDs, and trying to wrangle up idiot investors.
Whether or not some free energy schmuck puts "Open source" on their blog, doesn't mean they are altruistic. In fact, go through the history of these people and see they have been trying to make money the entire time. (And have been doing so shadily too.)
edit on 19-11-2013 by boncho because:
(no reason given)
reply to post by boncho
Next lesson should be conservation of energy and how you can't get more out of system than you put in.
www.bluffton.edu...
(edited: forgot what thread I was in)
Next lesson should be conservation of energy and how you can't get more out of system than you put in.
You cannot get more energy out of a system than the energy you put in plus the energy of the system.
www.bluffton.edu...
(edited: forgot what thread I was in)
edit on 19-11-2013 by boncho because: (no reason given)
Arbitrageur
www.heatpumpcentre.org...
Here’s the first sentence:
Heat pump performance
The heat delivered by a heat pump is theoretically the sum of the heat extracted from the heat source and the energy needed to drive the cycle.
Why doesn’t that say “difference between” instead of “sum of”?
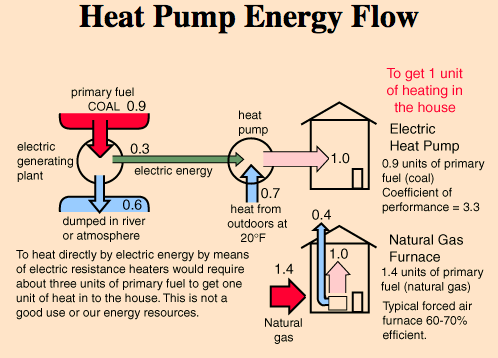
Mary, your initial diagram has 1kw of electricity going in (on the left), and 2kw of heat energy going in (from the top) and 3kw of heat energy going
out (to the right) - it's an open system, in that the input of the 2kw external heat energy is outside of the heat engine itself. But the sums add
up - 3kw in = 3kw out.
the heat out equals the sum of the heat in, plus the electricity in to produce more heat.
there's no magic here - it's not rocket surgery.
the heat out equals the sum of the heat in, plus the electricity in to produce more heat.
there's no magic here - it's not rocket surgery.
Mary Rose
Arbitrageur
www.heatpumpcentre.org...
Here’s the first sentence:
Heat pump performance
The heat delivered by a heat pump is theoretically the sum of the heat extracted from the heat source and the energy needed to drive the cycle.
Why doesn’t that say “difference between” instead of “sum of”?
In order to transport heat from a heat source to a heat sink, external energy is needed to drive the heat pump. Theoretically, the total heat delivered by the heat pump is equal to the heat extracted from the heat source, plus the amount of drive energy supplied. Electrically-driven heat pumps for heating buildings typically supply 100 kWh of heat with just 20-40 kWh of electricity. Many industrial heat pumps can achieve even higher performance, and supply the same amount of heat with only 3-10 kWh of electricity.
www.heatpumpcentre.org...
Don't forget "theoretically".
Back to first and second law:
If the First Law of Thermodynamics says you can't win, then the Second Law of Thermodynamics says you can't even break even. The First Law is essentially a statement of conservation of energy and asserts that you can't get more energy out of a heat engine than you put in. But the Second Law says that no heat engine can use all the heat produced by a fuel to do work. The Carnot cycle sets the ideal efficiency which can be obtained if there is no friction, mechanical losses, leakage, etc., but real machine efficiencies are much less.
And the difference between heat pumps and heat engines.
**
When comparing the performance of heat pumps, it is best to avoid the word "efficiency" which has a very specific thermodynamic definition. The term coefficient of performance (COP) is used to describe the ratio of useful heat movement per work input. Most vapor-compression heat pumps use electrically powered motors for their work input. However, in many vehicle applications, mechanical energy from an internal combustion engine provides the needed work.
hyperphysics.phy-astr.gsu.edu...
hyperphysics.phy-astr.gsu.edu...
edit on 19-11-2013 by boncho because: (no reason given)
reply to post by CrastneyJPR
I see.
Thanks.
So, the "heat delivered" doesn't have anything to do with net cost to the heat pump owner. I don't know why I was thinking that.
I see.
Thanks.
So, the "heat delivered" doesn't have anything to do with net cost to the heat pump owner. I don't know why I was thinking that.
edit on
11/19/13 by Mary Rose because: Revise my interpretation
CrastneyJPR
Mary, your initial diagram has 1kw of electricity going in (on the left), and 2kw of heat energy going in (from the top) and 3kw of heat energy going out (to the right) - it's an open system, in that the input of the 2kw external heat energy is outside of the heat engine itself.
Is "overunity" a proper term to apply to that scenario?
Apparently you do, because you're not satisfied with the term Coefficient of Performance and you want to call it something else?
Mary Rose
Who cares?
What difference does it make?
Arbitrageur
Apparently you do, because you're not satisfied with the term Coefficient of Performance and you want to call it something else?
Mary Rose
Who cares?
What difference does it make?
I think the overunity crowd she's reading confuses COP with efficiency, and think that anything with a COP > 1 is more than 100% efficient and thus overunity, which isn't true. Back to the top of the thread, I guess, where I said that COP wasn't efficiency and wasn't telling her what she seemed to think.
reply to post by Bedlam
Hi,
This is Kenneth, the creator of patent search extension Petapator. I have just came across your post regarding Petapator.
I apologize that Pepator does not perform up to your expectation. Would you kindly let me know how I should improve Petapator? Specifically, why do you like the old user interface than the new one?
Thanks
Kenneth
P.S. Sorry to interrupt this thread, but I cannot send private message.
Hi,
This is Kenneth, the creator of patent search extension Petapator. I have just came across your post regarding Petapator.
I apologize that Pepator does not perform up to your expectation. Would you kindly let me know how I should improve Petapator? Specifically, why do you like the old user interface than the new one?
Thanks
Kenneth
P.S. Sorry to interrupt this thread, but I cannot send private message.
I've enjoyed reading the responses to the OP as well as the ongoing discussion re: energy efficiency diagnosis. However, the biggest issue with the
heat pump is that it doesn't blow hot air. what i mean is that the air pumped out by heat pump is always less than 98.6 degrees f. even though a
heat pump will circulate air to the median temp of 78-82 degrees as set by thermostat, the heated air from the vents will always feel cold against the
skin. heat pumps are smart and efficient in areas like texas and the southwest. i know because my dad lives down south and has a heat pump. but up in
the northeast where the 5 months out of the year are fairly cold, the heat pump does not make it comfortable enough. gas furnace blows the hottest. i
know, most the conversation is going to the argument of possibly more energy output than what was initially input. never mind me.
reply to post by Petapator
It takes an amazingly, enormously long time to return a result. At first it was not that way, but lately it might take as many as 15-20 minutes to come back. If it does.
In the meantime, you get the spinny yellow Petapator wheel o' death in the top left corner.
That's why I can post so much here while searching. I've got plenty of 15 minute time slots.
Also why I've gone back to the text search from USPTO and Google's patent search.
It takes an amazingly, enormously long time to return a result. At first it was not that way, but lately it might take as many as 15-20 minutes to come back. If it does.
In the meantime, you get the spinny yellow Petapator wheel o' death in the top left corner.
That's why I can post so much here while searching. I've got plenty of 15 minute time slots.
Also why I've gone back to the text search from USPTO and Google's patent search.
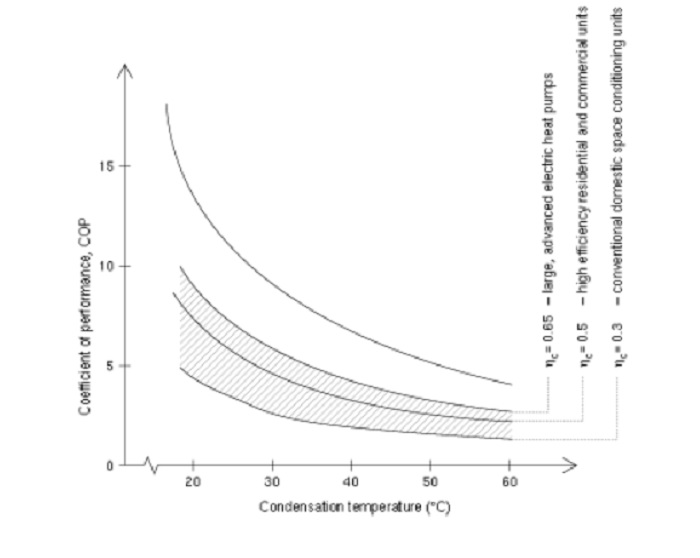
Arbitrageur
A much better measure is the Performance curve, which is not constrained to plus or minus 3 tenths of a degree etc, but shows the performance over a useful range of conditions. See in the attached link how the COP changes with temperature (performance curve) for the same heat pump, and for more explanation about the COP:
www.heatpumpcentre.org...
The performance curve still has COP on it:
So, where does overunity kick in on that curve?
edit on 11/21/13 by Mary Rose because: Trying to get rid of extraneous text generated by the systemextra DIV
new topics
-
BIDEN Admin Begins Planning For January 2025 Transition to a New President - Today is 4.26.2024.
2024 Elections: 1 hours ago -
Big Storms
Fragile Earth: 2 hours ago -
Where should Trump hold his next rally
2024 Elections: 5 hours ago -
Shocking Number of Voters are Open to Committing Election Fraud
US Political Madness: 5 hours ago -
Gov Kristi Noem Shot and Killed "Less Than Worthless Dog" and a 'Smelly Goat
2024 Elections: 6 hours ago -
Falkville Robot-Man
Aliens and UFOs: 6 hours ago -
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents: 7 hours ago -
Australian PM says the quiet part out loud - "free speech is a threat to democratic dicourse"...?!
New World Order: 8 hours ago -
Ireland VS Globalists
Social Issues and Civil Unrest: 8 hours ago -
Biden "Happy To Debate Trump"
2024 Elections: 9 hours ago
top topics
-
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents: 7 hours ago, 14 flags -
Blast from the past: ATS Review Podcast, 2006: With All Three Amigos
Member PODcasts: 11 hours ago, 13 flags -
Australian PM says the quiet part out loud - "free speech is a threat to democratic dicourse"...?!
New World Order: 8 hours ago, 12 flags -
Biden "Happy To Debate Trump"
2024 Elections: 9 hours ago, 12 flags -
Mike Pinder The Moody Blues R.I.P.
Music: 11 hours ago, 8 flags -
What is the white pill?
Philosophy and Metaphysics: 11 hours ago, 6 flags -
Shocking Number of Voters are Open to Committing Election Fraud
US Political Madness: 5 hours ago, 6 flags -
RAAF airbase in Roswell, New Mexico is on fire
Aliens and UFOs: 9 hours ago, 5 flags -
Ireland VS Globalists
Social Issues and Civil Unrest: 8 hours ago, 5 flags -
Where should Trump hold his next rally
2024 Elections: 5 hours ago, 5 flags
active topics
-
Mood Music Part VI
Music • 3112 • : underpass61 -
Big Storms
Fragile Earth • 10 • : AwakeNotWoke -
James O’Keefe: I have evidence that exposes the CIA, and it’s on camera.
Whistle Blowers and Leaked Documents • 12 • : pianopraze -
Australian PM says the quiet part out loud - "free speech is a threat to democratic dicourse"...?!
New World Order • 6 • : Scratchpost -
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest • 297 • : TheWoker -
BIDEN Admin Begins Planning For January 2025 Transition to a New President - Today is 4.26.2024.
2024 Elections • 5 • : AwakeNotWoke -
Where should Trump hold his next rally
2024 Elections • 18 • : TheMisguidedAngel -
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest • 15 • : marg6043 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 702 • : Crazierfox -
Putin, Russia and the Great Architects of the Universe
ATS Skunk Works • 28 • : lostgirl