It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
13
share:
A protein's function is determined by its shape - so two proteins with exactly the same amino acids but different shapes will do very different things. We know that proteins can misfold, become prions and cause disease - and that prion-like proteins control long-term memories. We also know that prions represent one type of epigenetic environmental response.
Fact is, proteins are cool. They can shape-shift. Probably do cartwheels. "BUT!" you say, "Each gene codes for a one particular protein with one specific structure, and just one predetermined biological function." ...Wrong. Since 2013, we have known that one of Ebola's key proteins -VP40- is a "transformer" protein - VP40 shape-shifts 3 times during assembly to fulfill 3 different functions. But is it a prion?
Scripps Research Institute Scientists Reveal How Deadly Ebola Virus Assembles
...“Like a ‘Transformer’, this protein of the Ebola virus adopts different shapes for different functions,” said Erica Ollmann Saphire, Ph.D., professor in the Department of Immunology and Microbial Science at TSRI. “It revises a central dogma of molecular biology—that a protein molecule has one shape that predestines one biological function."
...This “shape-shifting” or “transformer” behavior explains how the Ebola virus can control a multi-step viral lifecycle using only a very limited number of genes.
Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle
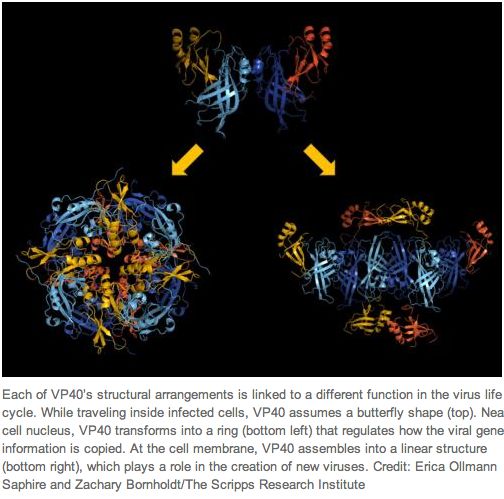
...VP40 rearranges into different structures, each with a distinct function required for the ebolavirus life cycle. A butterfly-shaped VP40 dimer traffics to the cellular membrane. Once there, electrostatic interactions trigger rearrangement of the polypeptide into a linear hexamer. These hexamers construct a multilayered, filamentous matrix structure that is critical for budding and resembles tomograms of authentic virions. A third structure of VP40, formed by a different rearrangement, is not involved in virus assembly but instead uniquely binds RNA to regulate viral transcription inside infected cells. These results provide a functional model for ebolavirus matrix assembly and the other roles of VP40 in the virus life cycle and demonstrate how a single wild-type, unmodified polypeptide can assemble into different structures for different functions.
edit on 5/7/14 by soficrow because: (no reason given)
Great article!
Seems most likely, it does appear possible.
It has become a crisis over in West Africa and other regions.
I am glad you posted this, the information is invaluable.
Seems most likely, it does appear possible.
It has become a crisis over in West Africa and other regions.
I am glad you posted this, the information is invaluable.
a reply to: ADVISOR
Thanks. ...I started paying attention to prions because I have fibromuscular dysplasia (FMD), but couldn't get any straight info from my medical people - so I had to research it myself. In the process I realized that the actin protein's role in FMD's fibrosis was most similar to Mad Cow prions. Initially, I viewed prions as really, really nasty disease agents. Now, not so much. My perspective changed as prion research became open source and more accessible, and I learned more.
Now, I believe we need to rethink biology, and embrace a new paradigm. Painful as that may be. ...One of the main obstacles is that research has to promise treatments and profits - doesn't leave a lot of room for learning, growing and changing paradigms.
Thanks. ...I started paying attention to prions because I have fibromuscular dysplasia (FMD), but couldn't get any straight info from my medical people - so I had to research it myself. In the process I realized that the actin protein's role in FMD's fibrosis was most similar to Mad Cow prions. Initially, I viewed prions as really, really nasty disease agents. Now, not so much. My perspective changed as prion research became open source and more accessible, and I learned more.
Now, I believe we need to rethink biology, and embrace a new paradigm. Painful as that may be. ...One of the main obstacles is that research has to promise treatments and profits - doesn't leave a lot of room for learning, growing and changing paradigms.
a reply to: rustyclutch
Thanks rustyclutch. But it's not just keeping up with Ebola - as Dr. Erica Ollmann Saphire says, "(the) structural rearrangements as seen in VP40 may be more common than previously thought."
...Seems clear we need to revise our understandings of prions, accept that they play a critical role in adaptation and evolution as well as normal biological functioning - and look a whole lot further than we have been looking.
Thanks rustyclutch. But it's not just keeping up with Ebola - as Dr. Erica Ollmann Saphire says, "(the) structural rearrangements as seen in VP40 may be more common than previously thought."
...Seems clear we need to revise our understandings of prions, accept that they play a critical role in adaptation and evolution as well as normal biological functioning - and look a whole lot further than we have been looking.
INDEED A BREAKTHROUGH OF SIGNIFIGANT PROPORTIONS....NOW IF THEY WILL JUST USE THE INFO FOR FIGHTING DISEASES INSTEAD OF GERM WARFARE.......
originally posted by: cavtrooper7
Anything look man made?
Genomic sequencing shows the current Ebola epidemic in West Africa is caused by a new strain that is NOT related to other Ebola strains in Africa - apparently, it evolved separately. And - researchers say the epidemic started with just ONE animal infecting only ONE human.
The results of full genetic sequencing suggest that the outbreak in Guinea isn't related to others that have occurred elsewhere in Africa, according to an international team that published its findings online in the New England Journal of Medicine (NEJM).
Emergence of Zaire Ebola Virus Disease in Guinea — Preliminary Report. New England Journal of Medicine, 2014; 140416140039002 DOI: 10.1056/NEJMoa1404505
….Full-length genome sequencing and phylogenetic analysis showed that EBOV from Guinea forms a separate clade in relationship to the known EBOV strains from the Democratic Republic of Congo and Gabon. …This study demonstrates the emergence of a new EBOV strain in Guinea.
"These results demonstrate that we are facing the emergence of a new "form" of this virus in Guinea," explains Hervé Raoul, Director of the BSL-4 Laboratory. This form is common to cases discovered since the month of December.
It would appear that the epidemic originated from a single introduction from animal to human.
...So why wasn't this new strain endemic in the area's bats and monkeys, at the least? Why just one infected animal?
This article from Global Policy Journal suggests guerilla bushmen terrorists have the ability to acquire contaminated samples and purposefully disseminate same to infect enemies without infecting themselves in the process. A laughably unlikely scenario, imho.
The Bioterrorist Threat of Ebola in East Africa and Implications for Global Health and Security
…The greater frequency with which Ebola is appearing raises questions about human accessibility to the virus and human usages of the virus for harmful purposes. The increase in natural outbreaks in the region, coupled with a possibility of a terrorist group recruiting experts to acquire the virus and to prepare it to use as a bioweapon, should lead policymakers to consider the risk of a deliberate outbreak. ...
However - it is both likely and plausible that corporate terrorists would spread Ebola to serve economic takeover(s) - they have the personnel, labs, expertise and motivation.
So why is the Global Policy Journal suggesting we "blame the native terrorists"? To cover up the fact that corporations are stepping up their takeover agenda. Obviously.
This paper deals with agricultural corporations but the analysis can be applied to corporations involved in resource extraction.
Agricultural Biowarfare and Bioterrorism
...Anti-agricultural biowarfare and bioterrorism differ significantly from the same activities directed against humans; for instance, there exist a variety of possibilities for economic gain for perpetrators, and the list of possible perpetrators includes corporations, which may have state-of-the-art technical expertise. Furthermore, attacks are substantially easier to do: the agents aren’t necessarily hazardous to humans; delivery systems are readily available and unsophisticated; maximum effect may only require a few cases; delivery from outside the target country is possible; and an effective attack can be constructed to appear natural. This constellation of characteristics makes biological attack on the agricultural sector of at least some countries a very real threat, perhaps more so than attack on the civilian population.
Agricultural corporations, including producers, processors, and shippers, could benefit immensely from the economic impacts, market share changes, and financial market effects of a successful biological attack. Many also employ expert plant pathologists or veterinarians and have large collections of pathogens. The combination of motivation, expertise, and materials within a single, closed organization is worrisome.
Also, one little fact gets glossed right over - Ebola is present in blood. Which means insect vectors. And - prions stay in the soil and water to be taken up by plants and ingested by animals. Ebola too is known to be sequestered in soil (at least). Check out dreamingawake's post for more info. Which means purposeful creation and dissemination of a new strain would be stupid in the extreme. Not that stupidity ever stopped these fools.
This VP40 protein takes on three different shapes to fulfill three different biological functions - and "revises a central dogma of molecular biology
- that a protein molecule has one shape that predestines one biological function."
Which raises an important question - how commonly do proteins shapeshift to fulfill biological functions in other creatures?
Which raises an important question - how commonly do proteins shapeshift to fulfill biological functions in other creatures?
originally posted by: soficrow
This VP40 protein takes on three different shapes to fulfill three different biological functions - and "revises a central dogma of molecular biology - that a protein molecule has one shape that predestines one biological function."
Which raises an important question - how commonly do proteins shapeshift to fulfill biological functions in other creatures?
Seems transformer proteins probably are fairly common. Just might explain a whole lot of diversity.
Transformer proteins
Transformer proteins (TFPs) are proteins that can transform from one conformation to a different one and simultaneously change their function.[1]
Protein structures in a given environment were thought to be defined completely by their amino acid sequence.[2] These protein structures are usually related to one single physiological protein activity. This hypothesis was, however, challenged by observations that proteins could fold in two alternative conformations, such as the prion proteins which exist in a physiologially active cellular form and an insoluble form.[3] Extending the concept of a protein that exists in a soluble and an insoluble form, for the bacterial transcription factor RfaH two entirely different structures were observed to coexist in solution.[4] RfaH is a two-domain protein, the C-terminal domain (CTD) of which can fold into alpha-helical and, alternatively, into beta-barrel form. These two interconvertible structures have two different functions, in alpha-helix form the CTD inhibits binding of the N-terminal domain to the RNA-polymerase, whereas the beta-barrel form recruits ribosomes. RfaH is thus the first member of the transformer protein class which obviously violates the original 'one sequence - one structure - one function' suggestion that governed protein research for decades .
PUBMED. Transformer proteins
a reply to: soficrow
This is rather interesting.
I know that muscle fibers can transform from one type to another in order to adapt to the stressors applied to it over time.
I may be confused here, but if genes code for protein synthesis, then do we have yet another example of how genetics are not the end all be all, in that this proteins, once synthesized, could fold and perform different functions within the organism?
So we have above, and beyond the gene working in ways to make us as we are.
Do we know the governing action of why these proteins fold into one structure or another? As in, does this appear to be "random" or dependent on environmental cues?
Excuse my ignorance if I scrambled up some things.
Okay, I read more. Prion protein seems to be within the CNS of healthy individuals, and refolds depending on environmental factors. So we've known of refolding proteins for some time, but it seems we gave this a distinction as being a "prion" period, and now know better. Now we know that more than one protein does this.
It does not seem to be a "mis"-folding, as the other instance which is discussed in this thread, has beneficial, yet different structure, and function from one fold to the next.
This leaves me exactly where I initially intuited about prions years ago... that being what seems to be dysfunction, is perhaps an alternative function which appears simply malignant, but may by a last-resort to curb yet a larger issue.
I'm not sure.
Prions form fibrous material called amyloid, much like a liver which has been too toxic, already gone through inflammation, and progressed into fibrosis.
I can't help but imagine that the refolding of these proteins are to form a cocoon around the perceived threat, when the immune system fails.
My last little bit will be to wonder WHY exactly prion disease is associated with animal eating it's own. I think of a pathogen that has already run it's course in one individual... it's been tagged by the immune system, attack mounted, and perhaps if virus lays dormant within tissue until it can remount an attack once the individual succumbs to debilitating life stressors.
Now this tissue is taken in by a same species member. Perhaps this pathogen is free to do it's thing without the immune system becoming activated, due to some tagging of the pathogen as already being dealt with?
We observe same species tissue being eaten, a refolding of protein, and assume it's the protein itself as the first cause, rather than a yet to be identified pathogen.
This is rather interesting.
I know that muscle fibers can transform from one type to another in order to adapt to the stressors applied to it over time.
I may be confused here, but if genes code for protein synthesis, then do we have yet another example of how genetics are not the end all be all, in that this proteins, once synthesized, could fold and perform different functions within the organism?
So we have above, and beyond the gene working in ways to make us as we are.
Do we know the governing action of why these proteins fold into one structure or another? As in, does this appear to be "random" or dependent on environmental cues?
Excuse my ignorance if I scrambled up some things.
Okay, I read more. Prion protein seems to be within the CNS of healthy individuals, and refolds depending on environmental factors. So we've known of refolding proteins for some time, but it seems we gave this a distinction as being a "prion" period, and now know better. Now we know that more than one protein does this.
It does not seem to be a "mis"-folding, as the other instance which is discussed in this thread, has beneficial, yet different structure, and function from one fold to the next.
This leaves me exactly where I initially intuited about prions years ago... that being what seems to be dysfunction, is perhaps an alternative function which appears simply malignant, but may by a last-resort to curb yet a larger issue.
I'm not sure.
Prions form fibrous material called amyloid, much like a liver which has been too toxic, already gone through inflammation, and progressed into fibrosis.
I can't help but imagine that the refolding of these proteins are to form a cocoon around the perceived threat, when the immune system fails.
My last little bit will be to wonder WHY exactly prion disease is associated with animal eating it's own. I think of a pathogen that has already run it's course in one individual... it's been tagged by the immune system, attack mounted, and perhaps if virus lays dormant within tissue until it can remount an attack once the individual succumbs to debilitating life stressors.
Now this tissue is taken in by a same species member. Perhaps this pathogen is free to do it's thing without the immune system becoming activated, due to some tagging of the pathogen as already being dealt with?
We observe same species tissue being eaten, a refolding of protein, and assume it's the protein itself as the first cause, rather than a yet to be identified pathogen.
edit on 9-7-2014 by pl3bscheese because: (no reason given)
originally posted by: pl3bscheese
a reply to: soficrow
.....do we have yet another example of how genetics are not the end all be all...?
Exactly. Great post, great questions. Really wish I had more time for you now but have to pick this up later for proper attention.
...My last little bit will be to wonder WHY exactly prion disease is associated with animal eating it's own.
Mostly misinformation for crowd control. ...The larger goal was/is to push genetics/eugenics, minimize the importance of other influences.
You might be interested in some general background info on "transformer proteins."
Transformer proteins
From Wikipedia, the free encyclopedia
Transformer proteins (TFPs) are proteins that can transform from one conformation to a different one and simultaneously change their function.[1]
Protein structures in a given environment were thought to be defined completely by their amino acid sequence.[2] These protein structures are usually related to one single physiological protein activity. This hypothesis was, however, challenged by observations that proteins could fold in two alternative conformations, such as the prion proteins which exist in a physiologially active cellular form and an insoluble form.[3] Extending the concept of a protein that exists in a soluble and an insoluble form, for the bacterial transcription factor RfaH two entirely different structures were observed to coexist in solution.[4] RfaH is a two-domain protein, the C-terminal domain (CTD) of which can fold into alpha-helical and, alternatively, into beta-barrel form. These two interconvertible structures have two different functions, in alpha-helix form the CTD inhibits binding of the N-terminal domain to the RNA-polymerase, whereas the beta-barrel form recruits ribosomes. RfaH is thus the first member of the transformer protein class which obviously violates the original 'one sequence - one structure - one function' suggestion that governed protein research for decades .
Cell Cycle. Dec 1, 2012; 11(23): 4289–4290.
Transformer proteins
Proteins are generally believed to adopt a unique fold, defined by their amino acid sequence, under specific environmental conditions.1 These unique structures, in turn, endow proteins with one specific function. However, not all proteins obey the “1 amino acid sequence → 1 fold → 1 function” scheme. ....
new topics
-
Kirkpatrick vs Fugal - Skinwalker ranch briefing.
Aliens and UFOs: 2 hours ago -
Lemon-aid and lime-aid
General Chit Chat: 3 hours ago -
Multipal Solar Storms Coming Our Way This Weekend
Fragile Earth: 3 hours ago -
US food sources declining becoming non edible .
Social Issues and Civil Unrest: 5 hours ago -
Journalism Against Judaism
Middle East Issues: 11 hours ago
top topics
-
Bibi’s Dilemma
Middle East Issues: 15 hours ago, 11 flags -
US food sources declining becoming non edible .
Social Issues and Civil Unrest: 5 hours ago, 5 flags -
Multipal Solar Storms Coming Our Way This Weekend
Fragile Earth: 3 hours ago, 5 flags -
Journalism Against Judaism
Middle East Issues: 11 hours ago, 4 flags -
Lemon-aid and lime-aid
General Chit Chat: 3 hours ago, 2 flags -
Kirkpatrick vs Fugal - Skinwalker ranch briefing.
Aliens and UFOs: 2 hours ago, 1 flags
active topics
-
Judge Postpones Trump Classified Docs Trial INDEFINITELY
US Political Madness • 173 • : Asher47 -
Pentagon UFO Hunter Reveals What He Knows About Aliens: Nothing
Aliens and UFOs • 16 • : Ophiuchus1 -
The Acronym Game .. Pt.3
General Chit Chat • 7819 • : bally001 -
Multipal Solar Storms Coming Our Way This Weekend
Fragile Earth • 10 • : VariedcodeSole -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 914 • : daskakik -
President Bidens Health is Declining Faster 5.8.2024 - He Should Stay Home.
2024 Elections • 29 • : budzprime69 -
Kirkpatrick vs Fugal - Skinwalker ranch briefing.
Aliens and UFOs • 1 • : NoCorruptionAllowed -
Lemon-aid and lime-aid
General Chit Chat • 6 • : randomtangentsrme -
Ooooh...it worked!!
Members • 25 • : LandofEnchantment -
Candidate TRUMP Now Has Crazy Judge JUAN MERCHAN After Him - The Stormy Daniels Hush-Money Case.
Political Conspiracies • 1452 • : CarlLaFong
13
