It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Entropy is rather hard to define, but can be best exemplified by the following example:
When an organism dies, it begins to decompose from an ordered biological structure back into the base molecular components.
In other words, organization or increased order is not favorable in chemical reactions. Order is therefore not capable of increasing over time according to the second law of thermodynamics.
Abiogenesis, which is the emergence of life from non-life, is therefore in direct opposition to this very well known thermodynamic law. A dead organism will always decay back into its base components given enough time. It does not work in reverse, which is essentially what abiogenesis is proposing.
For this reason, the emergence of life required some sort of Intelligence to implement the order exhibited in biological organisms.
Any homeowner has also realized this. Despite how much you build order, time will slowly destroy your efforts. This shows that intelligence is needed to create order, whereas time destroys order. This same logic therefore dismantles evolutionary theory as well, which supposed that over time increasing order was added to the genetic code.
No need to over-complicate it. Time does not create ordered biological beings from base components. It's a law of physics. The fact that our genetic code has persisted despite this law shows that the Intelligent spark that created us continues to this day.
When an organism dies, it begins to decompose from an ordered biological structure back into the base molecular components.
In other words, organization or increased order is not favorable in chemical reactions. Order is therefore not capable of increasing over time according to the second law of thermodynamics.
Abiogenesis, which is the emergence of life from non-life, is therefore in direct opposition to this very well known thermodynamic law. A dead organism will always decay back into its base components given enough time. It does not work in reverse, which is essentially what abiogenesis is proposing.
For this reason, the emergence of life required some sort of Intelligence to implement the order exhibited in biological organisms.
Any homeowner has also realized this. Despite how much you build order, time will slowly destroy your efforts. This shows that intelligence is needed to create order, whereas time destroys order. This same logic therefore dismantles evolutionary theory as well, which supposed that over time increasing order was added to the genetic code.
No need to over-complicate it. Time does not create ordered biological beings from base components. It's a law of physics. The fact that our genetic code has persisted despite this law shows that the Intelligent spark that created us continues to this day.
a reply to: cooperton
Well no. If you leave something like your dinner to decompose it first becomes alive.
Thermodynamics doesn't apply to all things alive, it's for gas and such.
Protein Synthesis on the other hand disproves all intelligent design because the highest law of life is to process one molecule into something 'orderly' as in reproducible.
Well no. If you leave something like your dinner to decompose it first becomes alive.
Thermodynamics doesn't apply to all things alive, it's for gas and such.
Protein Synthesis on the other hand disproves all intelligent design because the highest law of life is to process one molecule into something 'orderly' as in reproducible.
a reply to: cooperton
I have a book dating from the 60's. It was about a crisis in the genetics field that was trying with no success to redefine the words used, without implying an intelligence. Written by a geneticist.
Because if there is a code, there needs to be an intelligence that codes from somewhere.
I find interesting that many scientists that study quantum theory say that they went in atheists and are now believers in a higher conscience, since their researches show that for our realm to exist, "someone" from a higher dimension HAS to look at us.
I have a book dating from the 60's. It was about a crisis in the genetics field that was trying with no success to redefine the words used, without implying an intelligence. Written by a geneticist.
Because if there is a code, there needs to be an intelligence that codes from somewhere.
I find interesting that many scientists that study quantum theory say that they went in atheists and are now believers in a higher conscience, since their researches show that for our realm to exist, "someone" from a higher dimension HAS to look at us.
edit on 11-1-2022 by coamanach because:
(no reason given)
edit on 11-1-2022 by coamanach because: (no reason given)
Spend enough time on charnel grounds and or cemeteries for more wisdom in such matters.
When close to death it's typical that one either clings to more life or more materials sometimes both. Those clinging for more life enter it again almost immediately in the various sort of birth available away from the grosser elements. Those clinging to materials cling for who knows how long until dissolution occurs... the body is broken down or entropies.
Those that cling to both or see them in equanimity there is a co-operating cause. One is out of balance or goes to extremes and one is in balance knowing both are required.
What I find funny is going to a temple of "one robe one bowl" and there are many many many pairs of shoes at it as if everyone dove through the door into the place they felt safest. Well, the door cannot open, so how did they all get piled outside of it? I usually just straighten them all out and don't think about it or ask such questions.
So this is the first time bringing it up; Others that visit such places may be familiar with the phenomena of which I am speaking of. The same thing occurs with so called "expatriots" all grouping together in one place that reminds them of home, like flies on a corpse? It mainly has to do with food.
If one listens long enough? They will start to be driven "crazy" by their own language ...when that's all "contact" around the home "speaks" in... out of hundreds of languages? All my material nonsense has to speak in relative language to me ...as if I give a damn to hear it. Don't you know what the hell you are? Is all I would honestly have to say to it no matter what it said/says.
So it's obvious that more than material and the elements; that make up the body are what people become or are attached to... light and hearing is typical of more human like energies; blinded by light waves(visible spectrum) or deafened by sound waves(audible spectrum); they(not close to the human realm(ghosts)) tip toe around with their nose(blind and deaf) for whatever smells good; Like a dog... meaning there is also hunger and where there is hunger, there was at first thirst a craving or desire to creep around looking for food even from living people(think of Poltergeist "it bit me!")... if the ignorance of not knowing is bad? One should really contemplate on how knowing is even worse.
When close to death it's typical that one either clings to more life or more materials sometimes both. Those clinging for more life enter it again almost immediately in the various sort of birth available away from the grosser elements. Those clinging to materials cling for who knows how long until dissolution occurs... the body is broken down or entropies.
Those that cling to both or see them in equanimity there is a co-operating cause. One is out of balance or goes to extremes and one is in balance knowing both are required.
What I find funny is going to a temple of "one robe one bowl" and there are many many many pairs of shoes at it as if everyone dove through the door into the place they felt safest. Well, the door cannot open, so how did they all get piled outside of it? I usually just straighten them all out and don't think about it or ask such questions.
So this is the first time bringing it up; Others that visit such places may be familiar with the phenomena of which I am speaking of. The same thing occurs with so called "expatriots" all grouping together in one place that reminds them of home, like flies on a corpse? It mainly has to do with food.
If one listens long enough? They will start to be driven "crazy" by their own language ...when that's all "contact" around the home "speaks" in... out of hundreds of languages? All my material nonsense has to speak in relative language to me ...as if I give a damn to hear it. Don't you know what the hell you are? Is all I would honestly have to say to it no matter what it said/says.
So it's obvious that more than material and the elements; that make up the body are what people become or are attached to... light and hearing is typical of more human like energies; blinded by light waves(visible spectrum) or deafened by sound waves(audible spectrum); they(not close to the human realm(ghosts)) tip toe around with their nose(blind and deaf) for whatever smells good; Like a dog... meaning there is also hunger and where there is hunger, there was at first thirst a craving or desire to creep around looking for food even from living people(think of Poltergeist "it bit me!")... if the ignorance of not knowing is bad? One should really contemplate on how knowing is even worse.
edit on 11-1-2022 by Crowfoot because: editing
originally posted by: Peeple
a reply to: cooperton
Well no. If you leave something like your dinner to decompose it first becomes alive.
Thermodynamics doesn't apply to all things alive, it's for gas and such.
Protein Synthesis on the other hand disproves all intelligent design because the highest law of life is to process one molecule into something 'orderly' as in reproducible.
In chemistry (as in biochemistry) an orderly state is usually the state that conforms to the lowest viable energy level for the configuration.
a reply to: cooperton
False. Your analogies fail, and your analysis fails. Again. As always.
Entropy applies if no new energy is introduced into the system.
Homeowner understand this. If you put no energy into maintaining your home, it falls into disrepair, but if you do properly maintain your home it can last for a very long time indeed.
There are homes that are hundreds or even thousands of years old in daily use today as homes.
False. Your analogies fail, and your analysis fails. Again. As always.
Entropy applies if no new energy is introduced into the system.
Homeowner understand this. If you put no energy into maintaining your home, it falls into disrepair, but if you do properly maintain your home it can last for a very long time indeed.
There are homes that are hundreds or even thousands of years old in daily use today as homes.
edit on 11/1/2022 by rnaa because: (no reason
given)
originally posted by: rnaa
Entropy applies if no new energy is introduced into the system.
No, entropy is always a factor in all chemical equations. Entropy applies on the biochemical scale, not just the macro scale. Look up Gibbs free energy equation... "S" stands for entropy in the equation and it helps determine whether a reaction is favorable or not
Amino acid polymerization is energetically unfavorable in water, it is non-spontaneous. It's similar to lighting a match underwater. This is because amino acid decay is spontaneous in water, whereas amino acid synthesis is not. Reactions that increase entropy are more spontaneous than those that decrease entropy.
Do you expect a decayed leaf to turn back from dirt into a living leaf again? Because that's what you're arguing here.
You're ignoring common sense and basic biochemical laws to maintain your belief system.
edit on 11-1-2022 by cooperton because: (no reason
given)
a reply to: chr0naut
Sorry for the poor choice of words. Chemistry always was my nemesis, I didn't know I should read more.
I was just thinking something like 'rna where nature first experimented with molecules that got an inbuilt plan and function'
but I tried to sound smart
and failed
Sorry for the poor choice of words. Chemistry always was my nemesis, I didn't know I should read more.
I was just thinking something like 'rna where nature first experimented with molecules that got an inbuilt plan and function'
but I tried to sound smart
and failed
edit on 12-1-2022 by Peeple because: arrgh auto correct
originally posted by: Peeple
a reply to: Raggedyman
Mold and bacteria
So not my dinner, phew, you mean mould and bacteria abiogeneerate on my dinner, like in peanut butter jars?
a reply to: Raggedyman
It was an attempt to point out in a funny way that decomposing isn't just
as OP said.
There's a lot more happening.
It was an attempt to point out in a funny way that decomposing isn't just
will always decay back into its base components
as OP said.
There's a lot more happening.
originally posted by: Peeple
a reply to: cooperton
Well no. If you leave something like your dinner to decompose it first becomes alive.
Thermodynamics doesn't apply to all things alive, it's for gas and such.
Protein Synthesis on the other hand disproves all intelligent design because the highest law of life is to process one molecule into something 'orderly' as in reproducible.
Chemical systems overall go towards disorder, it's a thermodynamic law. Intelligent humans can invent and create ordered things against this disorder, but even these efforts have a net higher entropic by-product than the order that was created (imagine all the energy required to forge steel for example). That is why I know that biological beings were made by an intelligent Being, because chemical reactions acting randomly as supposed by abiogenesis and evolution would not physically be able to create an enduring ordered biochemical system.
You need an intelligent being to build ordered systems, because random chance chemical reactions will not increase order over time according to the 2nd law of thermodynamics.
edit on 12-1-2022 by cooperton because: (no reason given)
a reply to: cooperton
You can keep repeating that but it doesn't make it true.
It's not like out of nowhere there were suddenly complicated biological beings, they developed over time, first a cell who 'learned' something than a cell that got that code of the first cell and learned something new additionally etc.
There's just no trace of a creator-made being anywhere that doesn't carry the information of the first cell to be found.
And again (since you like to say the same thing over and over I'll do the same): thermodynamic (heat-energy) applies to gas and things and nothing else. Biological beings don't grow towards entropy, they got more complex, from the first cell to us. The building plan gets more complicated that's not entropy.
You age that's decay not entropy.
You decompose that's also not entropy, that's a system (Earth) in which the components circulate. But Earth won't melt into a puddle of gas just because you die.
You can keep repeating that but it doesn't make it true.
It's not like out of nowhere there were suddenly complicated biological beings, they developed over time, first a cell who 'learned' something than a cell that got that code of the first cell and learned something new additionally etc.
There's just no trace of a creator-made being anywhere that doesn't carry the information of the first cell to be found.
And again (since you like to say the same thing over and over I'll do the same): thermodynamic (heat-energy) applies to gas and things and nothing else. Biological beings don't grow towards entropy, they got more complex, from the first cell to us. The building plan gets more complicated that's not entropy.
You age that's decay not entropy.
You decompose that's also not entropy, that's a system (Earth) in which the components circulate. But Earth won't melt into a puddle of gas just because you die.
originally posted by: Peeple
a reply to: cooperton
You can keep repeating that but it doesn't make it true.
(biological beings) developed over time
You can keep repeating that, but it is by definition not possible as per the 2nd law of thermodynamics.
, first a cell who 'learned' something than a cell that got that code of the first cell and learned something new additionally etc.
How would the first cell form the multitude of protein and DNA macromolecules required for life if the formation of said protein and DNA polymers is remarkably unfavorable to occur, especially in water? This is what the entropic law insists upon... DNA and protein polymers tend to become monomers, not the other way around.
Biological beings don't grow towards entropy, they got more complex, from the first cell to us. The building plan gets more complicated that's not entropy.
Exactly, so this order among disorder is a hallmark of intelligent input. Just like when we intelligent humans create something that has an increased order, so too are biological beings a sign of being intelligently designed because they are created order in a system that destroys order over time.
You decompose that's also not entropy
No, biological decomposition is literally a textbook example of entropy. Decomposition of protein or nucleotide polymers to their monomeric form is a hallmark example of increasing entropy.
edit on 12-1-2022 by cooperton because: (no reason given)
Entropy increases in a CLOSED system. In an open system it can decrease.
edit on 12-1-2022 by 00018GE because: (no reason given)
originally posted by: 00018GE
Entropy increases in a CLOSED system. In an open system it can decrease.
But looking at the universe as a whole, there is an inevitable increasing entropy, so this doesn't make sense of how any order whatsoever could have emerged. Yet we have ordered cosmological orbits.. which allows the persistence of ordered biological beings. The more you pass on the entropic burden you eventually see that the entire universe required design. Because time, being burdened by inevitable entropy, cannot create the ordered systems exhibited in the cosmological and biological world.
The fact that there's order at all, despite a thermodynamic constant of increasing disorder, shows that order was implemented in the beginning.
edit on 12-1-2022 by cooperton because: (no reason given)
a reply to: cooperton
No. To all of this
It shows the laws of thermodynamics doesn't apply.
No. To all of this
But looking at the universe as a whole, there is an inevitable increasing entropy, so this doesn't make sense of how any order whatsoever could have emerged. Yet we have ordered cosmological orbits.. which allows the persistence of ordered biological beings. The more you pass on the entropic burden you eventually see that the entire universe required design. Because time, being burdened by inevitable entropy, cannot create the ordered systems exhibited in the cosmological and biological world.
The fact that there's order at all, despite a thermodynamic constant of increasing disorder, shows that order was implemented in the beginning.
It shows the laws of thermodynamics doesn't apply.
originally posted by: Peeple
It shows the laws of thermodynamics doesn't apply.
Look up Gibbs free energy equation. All biochemical reactions factor in the entropic change of a reaction. Reactions that generate more entropy are more spontaneous than reactions that don't.
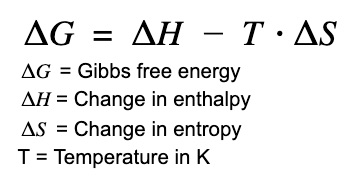
When "G" Is negative for a given reaction that means the reaction can occur spontaneously. If a given reaction has a large entropy increases, this causes an increase in spontaneity of the reaction. This is why decomposition of DNA and protein polymers is such a favorable reaction, because it has a notably large entropy for the reaction. Notice how as temperature increases, this makes G even more negative (i.e. more spontaneous!) So yeah the fact that DNA and protein polymerization has a negative entropy (i.e. increasing order) it is a notably unfavorable reaction. This is why when an organism dies it decomposes, rather than growing more legs or something absurd like that.
edit on 12-1-2022 by cooperton because: (no reason
given)
new topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 14 minutes ago -
Maestro Benedetto
Literature: 1 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 1 hours ago -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 5 hours ago -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 6 hours ago -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 6 hours ago -
The functionality of boldening and italics is clunky and no post char limit warning?
ATS Freshman's Forum: 7 hours ago -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 8 hours ago -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 8 hours ago -
Weinstein's conviction overturned
Mainstream News: 9 hours ago
top topics
-
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest: 11 hours ago, 9 flags -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics: 11 hours ago, 8 flags -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media: 14 hours ago, 7 flags -
Weinstein's conviction overturned
Mainstream News: 9 hours ago, 7 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 6 hours ago, 7 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 8 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 8 hours ago, 5 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 5 hours ago, 4 flags -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 14 minutes ago, 3 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 6 hours ago, 2 flags
active topics
-
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues • 95 • : Irishhaf -
Sunak spinning the sickness figures
Other Current Events • 23 • : NoCorruptionAllowed -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News • 14 • : WeMustCare -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 44 • : theshadowknows -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 233 • : DBCowboy -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 0 • : WeMustCare -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology • 31 • : glend -
Post A Funny (T&C Friendly) Pic Part IV: The LOL awakens!
General Chit Chat • 7135 • : underpass61 -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News • 62 • : ByeByeAmericanPie -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs • 72 • : yuppa
