It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
a reply to: Sparkymedic
www.gardenmyths.com...
Plants do not convert CO2 to oxygen. The oxygen produced by photosynthesis comes from splitting water. The CO2 is actually converted into sugars.
How much of that carbon dioxide was absorbed by plant life and converted to oxygen?
www.gardenmyths.com...
edit on 5/27/2021 by Phage because: (no reason given)
a reply to: Phage
So what about water vapor Phage, is it a gas or not?
Is evaporation a process that converts water into a gas? What of sublimation vs evaporation?
Are clouds, fog, steam, mists and vapors, are these gases or a suspension of particles?
Smoke and other particulates aren't gases obviously, an aerosol isn't a gas.
I can't seem to find H2O on this list of gases.
List of Gases - Wikipedia
So what about water vapor Phage, is it a gas or not?
Is evaporation a process that converts water into a gas? What of sublimation vs evaporation?
Are clouds, fog, steam, mists and vapors, are these gases or a suspension of particles?
Smoke and other particulates aren't gases obviously, an aerosol isn't a gas.
I can't seem to find H2O on this list of gases.
List of Gases - Wikipedia
edit on 27-5-2021 by MichiganSwampBuck because: Added extra
comments
a reply to: MichiganSwampBuck
Water vapor is a gas. An invisible gas.
Your source:
Water does not boil or sublime at these conditions.
Water vapor is a gas. An invisible gas.
I can't seem to find H2O on this list of gases.
Your source:
This is a list of gases at standard conditions, which means substances that boil or sublime at or below 25 °C (77 °F) and 1 atm pressure and are reasonably stable.
Water does not boil or sublime at these conditions.
edit on 5/27/2021 by Phage because: (no reason given)
originally posted by: Phage
a reply to: MichiganSwampBuck
Water vapor is a gas. An invisible gas.
I can't seem to find H2O on this list of gases.
Your source:
This is a list of gases at standard conditions, which means substances that boil or sublime at or below 25 °C (77 °F) and 1 atm pressure and are reasonably stable.
Water does not boil or sublime at these conditions.
Very well, thanks for the correction. I needed to hear it from you Phage to lay this to rest, not that I feel any smarter for the knowledge, but it's good to know. A matter of semantics I suppose, as I couldn't call oxygen a vapor, yet it can exist in a cloud form like water can.
originally posted by: Phage
a reply to: Sparkymedic
Plants do not convert CO2 to oxygen. The oxygen produced by photosynthesis comes from splitting water. The CO2 is actually converted into sugars.
How much of that carbon dioxide was absorbed by plant life and converted to oxygen?
www.gardenmyths.com...
Well, you learn something new everyday I guess.
That's pretty interesting.
That fact alone throws this theory I published in the OP somewhat upside down. Which is EXACTLY what I was looking for and why I appreciate ATS - someone can always punch a hole in something here.
I sincerely appreciate your input.
Still doesn't change my mind on the "green freaks" and their lunatic plans to tax the hell out of CO2. But that's just my opinion.
You can't call oxygen a vapor at room temperature because it's not a vapor at room temperature.
originally posted by: MichiganSwampBuck
Very well, thanks for the correction. I needed to hear it from you Phage to lay this to rest, not that I feel any smarter for the knowledge, but it's good to know. A matter of semantics I suppose, as I couldn't call oxygen a vapor, yet it can exist in a cloud form like water can.
Vapor has a specific technical definition, according to which oxygen vapor doesn't exist above about 155K or -115°C. But Oxygen at a temperature below -115°C you can certainly call a vapor, it is a vapor at say 150K.
This article talks about Oxygen vapor pressure below 154.78K:
Vapor pressure and fixed points of oxygen
Gaseous water happens to be a vapor at room temperature, but above 647 K (374 °C; 705 °F) technically it's no longer water vapor, like Oxygen is no longer oxygen vapor above about 154.78 K
a reply to: Arbitrageur
Yes, temperature and pressure are involved, just different stages for different compounds. Thanks all.
Yes, temperature and pressure are involved, just different stages for different compounds. Thanks all.
a reply to: Sparkymedic
Earth's temperature has changed drastically in the past without any nuclear industry. It is CO2 mainly.
The oceans absorb a lot of CO2, the reason why a lot of it is missing from our atmosphere. We have cut down a lot of forests so the ability of plant life to convert to O2 did not increase.
However, the oceans can absorb only so much, and eventually warming oceans will add to atmospheric CO2 and the amount of it will increase exponentially. It is possible that one-third of current land will get submerged and much of the balance may become inhabitable.
Earth's temperature has changed drastically in the past without any nuclear industry. It is CO2 mainly.
The oceans absorb a lot of CO2, the reason why a lot of it is missing from our atmosphere. We have cut down a lot of forests so the ability of plant life to convert to O2 did not increase.
However, the oceans can absorb only so much, and eventually warming oceans will add to atmospheric CO2 and the amount of it will increase exponentially. It is possible that one-third of current land will get submerged and much of the balance may become inhabitable.
a reply to: Gothmog
Unlikely. Why? Because petroleum and coal both contain telltale signs of their origins.
Science can be wrong sometimes but not every time.
Why we still have petroleum is because we continue to drill in new places at a fast pace.
It is hard to guess when petroleum and coal will run out; I think petroleum will taper off after 50 years and coal may last another 200 years.
The future of energy is sunshine.
Unlikely. Why? Because petroleum and coal both contain telltale signs of their origins.
Science can be wrong sometimes but not every time.
Why we still have petroleum is because we continue to drill in new places at a fast pace.
It is hard to guess when petroleum and coal will run out; I think petroleum will taper off after 50 years and coal may last another 200 years.
The future of energy is sunshine.
originally posted by: vedatruth1
a reply to: Gothmog
Unlikely. Why? Because petroleum and coal both contain telltale signs of their origins.
Science can be wrong sometimes but not every time.
Why we still have petroleum is because we continue to drill in new places at a fast pace.
It is hard to guess when petroleum and coal will run out; I think petroleum will taper off after 50 years and coal may last another 200 years.
The future of energy is sunshine.
1 ) What tell-tale signs ?
2 ) Science is often wrong.
3) What ?
4) There is evidence of oil-wells refilling.
5 ) How is sunshine the answer ? How about dead solar panels that contain dangerous materials ? How about failed batteries that contain more and even worse dangerous materials ?
And with that , I say adieu
a reply to: Sparkymedic
Global warming started before the invention of the nuclear reactor. It stated during the industrial revolution and got way worse after Wwii.
Global warming started before the invention of the nuclear reactor. It stated during the industrial revolution and got way worse after Wwii.
a reply to: Sparkymedic
Co2 isn't self correcting because human activity is causing it to be released faster than it can be absorbed.
More coal, oil and gas is being burned than Co2 is being stored
Co2 isn't self correcting because human activity is causing it to be released faster than it can be absorbed.
More coal, oil and gas is being burned than Co2 is being stored
Yes, there may be a self-correcting mechanism within certain limits, but human activity has overwhelmed it.
originally posted by: AaarghZombies
a reply to: Sparkymedic
Co2 isn't self correcting because human activity is causing it to be released faster than it can be absorbed.
More coal, oil and gas is being burned than Co2 is being stored
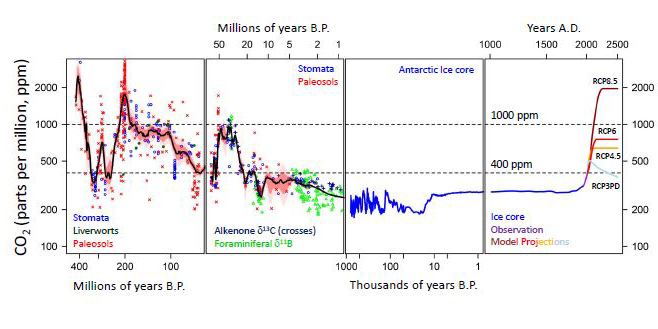
For most of the last thousand years, according to ice cores, CO2 seemed to be mostly a little under 300 ppm, maybe dipping down to 200 PPM then back up. It apparently never got up to the levels over 400 ppm we see today during those last 1000 years.
If you go further back to millions of years ago, ancient high CO2 levels declined for millions of years as CO2 was being sequestered into what are now fossil fuels.
Now, by burning those fossil fuels, we are releasing or "unsequestering" in only decades what took millions of years to sequester.
This is a graph showing the history of CO2 levels:
A 4.5 Billion-Year History of CO2 in our Atmosphere
new topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 1 hours ago -
Maestro Benedetto
Literature: 3 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 3 hours ago -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 6 hours ago -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 7 hours ago -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 8 hours ago -
The functionality of boldening and italics is clunky and no post char limit warning?
ATS Freshman's Forum: 9 hours ago -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 9 hours ago -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 10 hours ago -
Weinstein's conviction overturned
Mainstream News: 11 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 1 hours ago, 13 flags -
Krystalnacht on today's most elite Universities?
Social Issues and Civil Unrest: 13 hours ago, 9 flags -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media: 15 hours ago, 8 flags -
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics: 12 hours ago, 8 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 8 hours ago, 7 flags -
Weinstein's conviction overturned
Mainstream News: 11 hours ago, 7 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 10 hours ago, 7 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 9 hours ago, 5 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 6 hours ago, 4 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 7 hours ago, 3 flags
active topics
-
Supreme Court Oral Arguments 4.25.2024 - Are PRESIDENTS IMMUNE From Later Being Prosecuted.
Above Politics • 83 • : Sookiechacha -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 3 • : xuenchen -
Is AI Better Than the Hollywood Elite?
Movies • 10 • : 5thHead -
Sol Et Luna - TIME2024
Short Stories • 10 • : BrotherKinsMan -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 263 • : cherokeetroy -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest • 13 • : ToneD -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 26 • : nugget1 -
British TV Presenter Refuses To Use Guest's Preferred Pronouns
Education and Media • 168 • : Annee -
Manly P. Hall says Freemasonry is a religion?
Secret Societies • 22 • : Therealbeverage -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry • 11 • : Therealbeverage
