It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Or, The hot water evaporates at a faster rate than the cold water. Therefore its volume decreases more rapidly than that of the cold water. Which
means less volume to freeze than in the case of the cold water. Therefore, it freezes faster.
The problem I see with that is it can't be both hot and supercooled at the same time. If it's evaporating more when it's hot anything falling back in won't do anything to the still hot water.
originally posted by: swanne
a reply to: Arbitrageur
Ooh, I've got an idea. Maybe the evaporation of hot water solidifies upon contact with the cold of the freezer, as meanwhile the main body of liquid reaches a supercooled state. When the frozen crystals from evaporation fall back on the supercooled fluid, they trigger an avalanche and the supercooled turns to ice.
Also as explained in the video, these experiments have been replicated with closed top containers, which rules out the idea of something falling in along with some other variables.
a reply to: seasonal
Agreed. people believe the dumbdest stuff.
The people that promote crap like this want us to disbelieve science. That way you can be led to believe anything. After all science is flawed, see?
(put hot water in the freezer and cold too) I have and cold freezes faster.
Agreed. people believe the dumbdest stuff.
The people that promote crap like this want us to disbelieve science. That way you can be led to believe anything. After all science is flawed, see?
a reply to: Arbitrageur
Closed top? Which implies higher rate of condensation... A higher rate of heat dissipation...
But then, the container is closed and heat cannot escape. Damn, you're right, solves nothing. Well, was worth a try.
Closed top? Which implies higher rate of condensation... A higher rate of heat dissipation...
But then, the container is closed and heat cannot escape. Damn, you're right, solves nothing. Well, was worth a try.
a reply to: Arbitrageur
First thing that comes to mind upon reading this is that warmer water is closer to the gaseous state. The cold gas molecules of the freezer i would imagine more readily absorb into the warmer water than the colder due to the warmer water being closer to gas state. Combined with cold sinking, warm rising dynamic.
Essentially the warmer water is more permeable to the freezer gasses.
*shrug
First thing that comes to mind upon reading this is that warmer water is closer to the gaseous state. The cold gas molecules of the freezer i would imagine more readily absorb into the warmer water than the colder due to the warmer water being closer to gas state. Combined with cold sinking, warm rising dynamic.
Essentially the warmer water is more permeable to the freezer gasses.
*shrug
a reply to: Arbitrageur
The conditions need to be identical for the experiment to be without question. Somebody here will tear it apart if both the hot and cold sample aren't in the exact same conditions while waiting to observe the outcome.
The conditions need to be identical for the experiment to be without question. Somebody here will tear it apart if both the hot and cold sample aren't in the exact same conditions while waiting to observe the outcome.
Did you watch the video in the OP? That is one of the hypotheses considered.
originally posted by: swanne
Or, The hot water evaporates at a faster rate than the cold water. Therefore its volume decreases more rapidly than that of the cold water. Which means less volume to freeze than in the case of the cold water. Therefore, it freezes faster.
a reply to: Arbitrageur
Oh.
No, I haven't watched the video yet (I'm on a videographically-challenged device).
Oh.
No, I haven't watched the video yet (I'm on a videographically-challenged device).
This is hardly an anti-science thread, in fact I'm usually trying to debunk anti-science threads. I didn't attempt to replicate the results of the experimenter who got the hot water to freeze faster in 28 out of 28 trials, but his proposed explanation for the experimental results he observed are consistent with known scientific principles even if they might seem to disagree with an overly simplified thermodynamic analysis.
originally posted by: intrptr
Agreed. people believe the dumbdest stuff.
The people that promote crap like this want us to disbelieve science. That way you can be led to believe anything. After all science is flawed, see?
a reply to: Arbitrageur
A 'fridge with a freezer section blows the cold air from the compressor into the unit from that freezer vent. Usually, there is a fan to help distribute that colder air. Then that air is channeled into the fresh food section because it has taken up some heat from the frozen food.
you gotta know the scientific parameters of your experiments before you can expound on a theory.
A 'fridge with a freezer section blows the cold air from the compressor into the unit from that freezer vent. Usually, there is a fan to help distribute that colder air. Then that air is channeled into the fresh food section because it has taken up some heat from the frozen food.
you gotta know the scientific parameters of your experiments before you can expound on a theory.
edit on 9-2-2017 by Aliensun
because: (no reason given)
Hot water will freeze at a faster rate.
If your "hot" water is say, 80 degrees and your "cold" water is 50 degrees, the hot water will reach 70 degrees before your cold water reaches 40 degrees.
The hot water has cooled off by 10 degrees much faster than the cold water has cooled by 10 degrees.
However, while your hot water is freezing "faster" (at a greater rate) than your cold water, the hot water will never catch up with the cold water and the cold water will still be frozen first.
As the hot water grows cooler the rate at which it cools slows down.
If your "hot" water is say, 80 degrees and your "cold" water is 50 degrees, the hot water will reach 70 degrees before your cold water reaches 40 degrees.
The hot water has cooled off by 10 degrees much faster than the cold water has cooled by 10 degrees.
However, while your hot water is freezing "faster" (at a greater rate) than your cold water, the hot water will never catch up with the cold water and the cold water will still be frozen first.
As the hot water grows cooler the rate at which it cools slows down.
Just wanna throw this out there, everything discussed so far relates to the different states, of course temperature is just a fancy term for measuring
the excitation of the molecules.
Not sure how this would relate in any way, but I believe even at its coldest recorded the molecules still exhibit some form of movement, in that they tried to get to an absolute standstill....they came close, but not absolute.
Is there any way the faster the molecules are moving could exhibit a paradoxical effect by cooling down faster?
Completely counter-intuitive, nonsensical, my kind of crazy ass crazy and layman's logic...sorry this is the best I can contribute.
Flame me softly
Not sure how this would relate in any way, but I believe even at its coldest recorded the molecules still exhibit some form of movement, in that they tried to get to an absolute standstill....they came close, but not absolute.
Is there any way the faster the molecules are moving could exhibit a paradoxical effect by cooling down faster?
Completely counter-intuitive, nonsensical, my kind of crazy ass crazy and layman's logic...sorry this is the best I can contribute.
Flame me softly
a reply to: solargeddon
If you were laser cooling an out of phase sample there might be an asymptotic response.
If you were laser cooling an out of phase sample there might be an asymptotic response.
That makes perfect sense.
originally posted by: abe froman
Hot water will freeze at a faster rate.
If your "hot" water is say, 80 degrees and your "cold" water is 50 degrees, the hot water will reach 70 degrees before your cold water reaches 40 degrees.
The hot water has cooled off by 10 degrees much faster than the cold water has cooled by 10 degrees.
That's what we might expect and is what I observed in my experiments, however others have found this to not consistently be the case so I'm not sure we can say the cold water will always freeze first. Sometimes it does and sometimes it doesn't, according to experiments other than my own.
However, while your hot water is freezing "faster" (at a greater rate) than your cold water, the hot water will never catch up with the cold water and the cold water will still be frozen first.
a reply to: Arbitrageur
First: I haven't watched the video (being at work). That said, do any of the experiments include a time series of water temperatures?
First: I haven't watched the video (being at work). That said, do any of the experiments include a time series of water temperatures?
a reply to: Phage
The video doesn't have that kind of detail but the paper I posted on page one has graphs like this one but I'm not sure if this is what you mean:
A search for the Mpemba effect: When hot water freezes faster then cold water
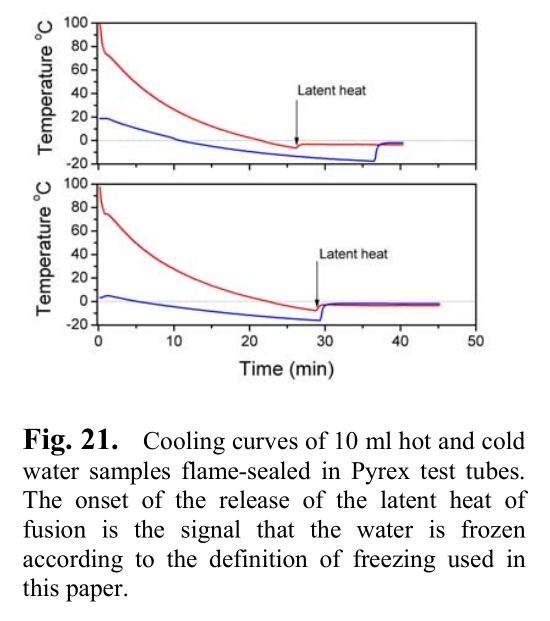
Looks like the top graph shows the hot water freezing first according to the definition of freezing used in this paper.
The video doesn't have that kind of detail but the paper I posted on page one has graphs like this one but I'm not sure if this is what you mean:
A search for the Mpemba effect: When hot water freezes faster then cold water

Looks like the top graph shows the hot water freezing first according to the definition of freezing used in this paper.
originally posted by: Arbitrageur
This is hardly an anti-science thread, in fact I'm usually trying to debunk anti-science threads. I didn't attempt to replicate the results of the experimenter who got the hot water to freeze faster in 28 out of 28 trials, but his proposed explanation for the experimental results he observed are consistent with known scientific principles even if they might seem to disagree with an overly simplified thermodynamic analysis.
originally posted by: intrptr
Agreed. people believe the dumbdest stuff.
The people that promote crap like this want us to disbelieve science. That way you can be led to believe anything. After all science is flawed, see?
I know you are smarter than that. Keep it simple, whats wrong wth this statement?
Cold stuff cools down faster than hot stuff does.
And I don't mean that to insult you, I respect you and your content here, just surprised you would be tangled by some guy claiming he proved it again and again, defying physics, in this case.
new topics
-
Biden pardons 39 and commutes 1500 sentences…
Mainstream News: 31 minutes ago -
Jan 6th truth is starting to leak out.
US Political Madness: 1 hours ago -
Deep state control - How your tax dollars are used to censor and brainwash
Propaganda Mill: 2 hours ago -
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News: 2 hours ago -
Top Sci Fi/Horror Crossover Movies
Movies: 5 hours ago -
Magic Vaporizing Ray Gun Claim - More Proof You Can't Believe Anything Hamas Says
War On Terrorism: 6 hours ago -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies: 6 hours ago -
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness: 7 hours ago
top topics
-
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness: 7 hours ago, 17 flags -
Jan 6th truth is starting to leak out.
US Political Madness: 1 hours ago, 13 flags -
Magic Vaporizing Ray Gun Claim - More Proof You Can't Believe Anything Hamas Says
War On Terrorism: 6 hours ago, 6 flags -
Top Sci Fi/Horror Crossover Movies
Movies: 5 hours ago, 6 flags -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies: 6 hours ago, 5 flags -
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News: 2 hours ago, 5 flags -
What Am I Hearing
General Chit Chat: 15 hours ago, 4 flags -
Deep state control - How your tax dollars are used to censor and brainwash
Propaganda Mill: 2 hours ago, 1 flags -
Biden pardons 39 and commutes 1500 sentences…
Mainstream News: 32 minutes ago, 0 flags
active topics
-
Biden pardons 39 and commutes 1500 sentences…
Mainstream News • 3 • : Xtrozero -
Will all hell break out? Jersey drones - blue beam
Aliens and UFOs • 52 • : network dude -
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News • 13 • : WeMustCare -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies • 16 • : Lazy88 -
President-Elect DONALD TRUMP's 2nd-Term Administration Takes Shape.
Political Ideology • 317 • : network dude -
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness • 18 • : network dude -
FBI Director CHRISTOPHER WRAY Will Resign Before President Trump Takes Office on 1.20.2025.
US Political Madness • 22 • : VariedcodeSole -
Jan 6th truth is starting to leak out.
US Political Madness • 9 • : WeMustCare -
The Fight for Election Integrity Continues -- Audits, Criminal Investigations, Legislative Reform
2024 Elections • 4365 • : IndieA -
Magic Vaporizing Ray Gun Claim - More Proof You Can't Believe Anything Hamas Says
War On Terrorism • 9 • : FullHeathen
