It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
There is an article at the website Live Science about work done by researchers at the University of Munich in Germany. The article is entitled
"Atoms Reach Record Temperature, Colder than Absolute Zero":
The findings of the researchers appear in the journal Science in the 4 January 2013 issue. Here is the Abstract:
"We have created the first negative absolute temperature state for moving particles," said researcher Simon Braun at the University of Munich in Germany.
The findings of the researchers appear in the journal Science in the 4 January 2013 issue. Here is the Abstract:
Absolute temperature is usually bound to be positive. Under special conditions, however, negative temperatures—in which high-energy states are more occupied than low-energy states—are also possible. Such states have been demonstrated in localized systems with finite, discrete spectra. Here, we prepared a negative temperature state for motional degrees of freedom. By tailoring the Bose-Hubbard Hamiltonian, we created an attractively interacting ensemble of ultracold bosons at negative temperature that is stable against collapse for arbitrary atom numbers. The quasimomentum distribution develops sharp peaks at the upper band edge, revealing thermal equilibrium and bosonic coherence over several lattice sites. Negative temperatures imply negative pressures and open up new parameter regimes for cold atoms, enabling fundamentally new many-body states.
This was also reported on ScienceDaily. com aka, the greatest website on the internet.
A Temperature Below Absolute Zero: Atoms at Negative Absolute Temperature Are the Hottest Systems in the World
www.sciencedaily.com...
With out completely thinking this through, I will state the following lol.
This is some amazing stuff right here. If harnessed and used correctly, it would seem that transitioning atoms into this negatively valued temperature, would provide more energy than said atom could produce while being at a 'positive' temperature. I wonder what it takes to keep these atoms at these temperatures, and the energy required to achieve these negative temps. Maybe, this is the key to alternative fuel sources!
Like I said.... "with out completely thinking this through"
deny ignorance.
Alright, I don't feel like an idiot lol. After reading the entire article on sciencedaily, I came to this part:
A Temperature Below Absolute Zero: Atoms at Negative Absolute Temperature Are the Hottest Systems in the World
www.sciencedaily.com...
“The inverted Boltzmann distribution is the hallmark of negative absolute temperature; and this is what we have achieved,” says Ulrich Schneider. “Yet the gas is not colder than zero kelvin, but hotter,” as the physicist explains: “It is even hotter than at any positive temperature – the temperature scale simply does not end at infinity, but jumps to negative values instead.”
With out completely thinking this through, I will state the following lol.
This is some amazing stuff right here. If harnessed and used correctly, it would seem that transitioning atoms into this negatively valued temperature, would provide more energy than said atom could produce while being at a 'positive' temperature. I wonder what it takes to keep these atoms at these temperatures, and the energy required to achieve these negative temps. Maybe, this is the key to alternative fuel sources!
Like I said.... "with out completely thinking this through"
deny ignorance.
Alright, I don't feel like an idiot lol. After reading the entire article on sciencedaily, I came to this part:
Matter at negative absolute temperature has a whole range of astounding consequences: with its help, one could create heat engines such as combustion engines with an efficiency of more than 100%. This does not mean, however, that the law of energy conservation is violated. Instead, the engine could not only absorb energy from the hotter medium, and thus do work, but, in contrast to the usual case, from the colder medium as well.
At purely positive temperatures, the colder medium inevitably heats up in contrast, therefore absorbing a portion of the energy of the hot medium and thereby limits the efficiency. If the hot medium has a negative temperature, it is possible to absorb energy from both media simultaneously. The work performed by the engine is therefore greater than the energy taken from the hotter medium alone – the efficiency is over 100 percent.
edit on 15-1-2013 by retirednature because: additional comment
reply to post by retirednature
I learned about this from listening to a Red Ice Radio interview of Jeremy Rys - Alien Scientist. It is interesting to listen to his reaction to the news.
I learned about this from listening to a Red Ice Radio interview of Jeremy Rys - Alien Scientist. It is interesting to listen to his reaction to the news.
The title from Live Science is somewhat misleading. "Negative temperature" is not about producing temperatures below absolute zero. The key is in this
quote from the original article:
The experiment involved increasing the energy state of the atoms. A bit more understandably stated here:
scienceblogs.com...
Under special conditions, however, negative temperatures—in which high-energy states are more occupied than low-energy states—are also possible.
The experiment involved increasing the energy state of the atoms. A bit more understandably stated here:
But given that I did in fact get to that population inversion, it formally acts like a negative temperature system in that it has more excited atoms than are possible with any finite positive temperature. Negative temperatures are thus in essence really really hot.
scienceblogs.com...
edit on 1/15/2013 by Phage because: (no reason given)
This could lead to some inventions that would be well suited in space travel, it`s gets pretty cold out there n space, right?
Originally posted by Mary Rose
The article is entitled "Atoms Reach Record Temperature, Colder than Absolute Zero"
Originally posted by Phage
The title from Live Science is somewhat misleading. "Negative temperature" is not about producing temperatures below absolute zero.
Originally posted by Phage
A bit more understandably stated here . . .
scienceblogs.com...
The title of the Science Blogs article is "Less Than Absolute Zero." So, are you saying that less than is different from colder than?
reply to post by Mary Rose
scienceblogs.com...
Sort of. A negative number is less than zero but a negative temperature is not colder than absolute zero. I'm saying that there is no temperature colder than absolute zero. The author of the blog is correcting the misunderstandings that such a temperature was produced. The title is a goof on the movie title "Less than Zero".
The title of the Science Blogs article is "Less Than Absolute Zero." So, are you saying that less than is different from colder than?
But don’t worry – in the usual “average energy of the atoms at thermal equilibrium” sense, absolute zero remains the coldest possible temperature.
scienceblogs.com...
edit on 1/15/2013 by Phage because: (no reason given)
Originally posted by Mary Rose
There is an article at the website Live Science about work done by researchers at the University of Munich in Germany. The article is entitled "Atoms Reach Record Temperature, Colder than Absolute Zero":
Absolute zero is often thought to be the coldest temperature possible. But now researchers show they can achieve even lower temperatures for a strange realm of "negative temperatures."
Oddly, another way to look at these negative temperatures is to consider them hotter than infinity, researchers added. . . .
reply to post by Mary Rose
Yes. I know. It is a terribly written article. For one thing, it doesn't explain the difference between the "temperature" recorded by a thermometer and the "temperature" in the experiment.
The one I posted explains it much more clearly. The temperature is not less than absolute zero.
Yes. I know. It is a terribly written article. For one thing, it doesn't explain the difference between the "temperature" recorded by a thermometer and the "temperature" in the experiment.
The one I posted explains it much more clearly. The temperature is not less than absolute zero.
edit on 1/15/2013 by Phage because: (no reason given)
Originally posted by Mary Rose
"We have created the first negative absolute temperature state for moving particles," said researcher Simon Braun at the University of Munich in Germany.
The other quotes from the researchers in the article are:
and
"The inverted Boltzmann distribution is the hallmark of negative absolute temperature, and this is what we have achieved," said researcher Ulrich Schneider, a physicist at the University of Munich in Germany. "Yet the gas is not colder than zero kelvin, but hotter. It is even hotter than at any positive temperature — the temperature scale simply does not end at infinity, but jumps to negative values instead."
"The temperatures we achieved are negative nanokelvin," Schneider told LiveScience.
The title of the paper in the journal Science is "Negative Absolute Temperature for Motional Degrees of Freedom."
reply to post by Phage
Thanks for the link. Certainly a much better explanation!
I checked out other articles by Matt Springer, who wrote that one. Good stuff!
Thanks for the link. Certainly a much better explanation!
I checked out other articles by Matt Springer, who wrote that one. Good stuff!
From the PHYS ORG article "Atoms at negative absolute temperature:
The hottest systems in the world":
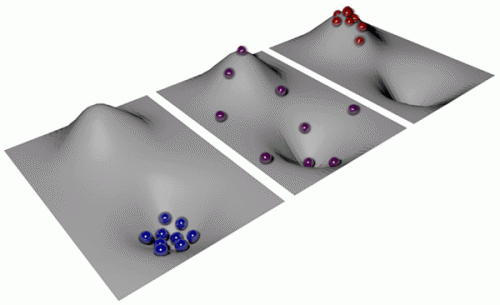
Fig. 1
. . . The underlying principle can best be visualized with an illustration (see Fig. 1): If one starts at positive temperatures (left figure) and increases the total energy of the balls by heating them up, the balls will also spread into regions of high energy. If one heated the balls to infinite temperature (central figure), each point in the landscape would be equally probable, irrespective of its energy. If one could add even more energy and thereby heat the balls even further, the balls would preferably gather at high-energy states (right figure) and would be even hotter than at infinite temperature. The Boltzmann distribution would be inverted, and the temperature therefore negative. At first sight it may sound strange that a negative absolute temperature is hotter than a positive one. This is, however, simply a consequence of the historic definition of absolute temperature; if it were defined differently, this apparent contradiction would not exist.
From "Atoms at negative absolute temperatures":
. . .Of course, this realisation of negative absolute temperatures doesn’t mean that absolute zero can be reached. It is a completely different challenge to slow down atomic motions towards zero than it is to change the overall energy distribution of the ensemble. Still, these are intriguing thermodynamical systems, and there is plenty to study about the implications of such reversed energy distributions.
reply to post by Mary Rose
Very good. Now read and understand this part of that article.
phys.org...
The temperature is not below absolute zero. You can refer to this article from 2010.
In a manner of speaking, this is similar to the discussions about light exceeding the speed of light.
Very good. Now read and understand this part of that article.
At first sight it may sound strange that a negative absolute temperature is hotter than a positive one. This is, however, simply a consequence of the historic definition of absolute temperature; if it were defined differently, this apparent contradiction would not exist.
phys.org...
The temperature is not below absolute zero. You can refer to this article from 2010.
www.phys.ncku.edu.tw...
Under certain conditions, a closed system can be described by a negative temperature, and, surprisingly, be hotter than the same system at any positive temperature. This article describes how it all works.
In a manner of speaking, this is similar to the discussions about light exceeding the speed of light.
edit on 1/15/2013 by Phage because: (no
reason given)
Originally posted by Phage
The temperature is not below absolute zero.
I didn't say it was.
No need to repeat yourself.
reply to post by Mary Rose
Since you made no comment it wasn't clear what you were saying...or thinking.
Since you made no comment it wasn't clear what you were saying...or thinking.
edit on 1/15/2013 by Phage because: (no reason given)
reply to post by Phage
I made no comment whatsoever. I chose what to quote from the article. And you turned around and re-quoted part of my quote from the article. Entirely unnecessary.
I made no comment whatsoever. I chose what to quote from the article. And you turned around and re-quoted part of my quote from the article. Entirely unnecessary.
reply to post by Mary Rose
I see.
An external quote with no comment of your own.
Tell us Mary, what do you think about what that quote says?
I see.
An external quote with no comment of your own.
Tell us Mary, what do you think about what that quote says?
edit on 1/15/2013 by Phage because: (no reason given)
reply to post by Phage
That the obsession with temperature is a technicality and the important thing is the implications of the work done by the researchers.
I will be looking for comments from scientists about those implications.
That the obsession with temperature is a technicality and the important thing is the implications of the work done by the researchers.
I will be looking for comments from scientists about those implications.
reply to post by Mary Rose
I'm not sure there is much in the way of practical implications since the conditions under which this was accomplished are quite contrived and it is a specialized case of what has been accomplished previously. It would seem to be more of a confirmation of theory than anything else.
But who knows, science for the sake of science usually leads somewhere else.
I'm not sure there is much in the way of practical implications since the conditions under which this was accomplished are quite contrived and it is a specialized case of what has been accomplished previously. It would seem to be more of a confirmation of theory than anything else.
But who knows, science for the sake of science usually leads somewhere else.
edit on 1/15/2013 by Phage because: (no reason given)
new topics
-
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 4 hours ago -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 9 hours ago -
Maestro Benedetto
Literature: 11 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 11 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 9 hours ago, 28 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 16 hours ago, 8 flags -
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 4 hours ago, 8 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 15 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 17 hours ago, 5 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 15 hours ago, 4 flags -
Is AI Better Than the Hollywood Elite?
Movies: 11 hours ago, 3 flags -
The functionality of boldening and italics is clunky and no post char limit warning?
ATS Freshman's Forum: 17 hours ago, 1 flags -
Maestro Benedetto
Literature: 11 hours ago, 1 flags
active topics
-
A Warning to America: 25 Ways the US is Being Destroyed
New World Order • 6 • : theatreboy -
Supreme Court to decide if states can control fate of social media
Education and Media • 14 • : SMMPanelPro -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 35 • : Lazy88 -
Definitive 9.11 Pentagon EVIDENCE.
9/11 Conspiracies • 426 • : Lazy88 -
Alternate Electors vs Fake Electors - What is the Difference.
2024 Elections • 117 • : ADVISOR -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 691 • : Imbackbaby -
Victoria government has cancelled the commmonwealth games, no money.
Regional Politics • 3 • : nazaretalazareta -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 19 • : ADVISOR -
When an Angel gets his or her wings
Religion, Faith, And Theology • 22 • : AcrobaticDreams1 -
King Charles 111 Diagnosed with Cancer
Mainstream News • 321 • : FlyersFan