It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: M5xaz
originally posted by: Vector99
a reply to: liejunkie01
A simple look at the planet Venus debunks your entire thread, sorry.
Venus 's atmosphere is 96.5% CO2.
Earth's atmosphere is 400 PARTS PER MILLION
Simple FACTS debunk your entire, uninformed, response.
It's good to get that out of the way from the start: The Earth is in no danger of becoming like Venus.
However, it has had much of its ice caps melt quite a many times in its history, causing ocean levels to rise and fall, and causing climate in various regions to go from desert to tropical, and back.
That stuff will cause harm to people. Real people who actually exist, and who matter. Not just imaginary people.
originally posted by: Outlier13
a reply to: mbkennel
Mate...seriously...stop drinking the kool-aid. You apparently didn't see my initial post in this thread which had to do with critical thinking. That first article you linked IMMEDIATELY discredits itself within the second sentence. "Land use" is the key phrase.
Do you have any idea how much of the actual surface of this planet man inhabits let alone on an industrial scale? Less than 1%. How does man occupying less than 1% of the habitable land on the planet contribute to 40% of global warming?
Honestly, I want to know if you think this is even remotely logical?
Mankind makes use of much more than 1% of the Earth. Have ever flown in an airplane over the USA and looked out the window? It's basically a giant patchwork quilt of farms. A few cities. A few reserves that are part of the park system. But mostly farms.
But land use isn't the issue for CO2. The problem is that we are releasing more CO2 than the plant life is able to recapture.
Those "fossil fuels" are giant reserves of captured CO2. If we release it, then it's like spending your life savings. It took millions of years to capture all that carbon underground.
CO2 itself only contributes a slight bit to the temperature, but it sets a feedback effect in motion. Water moisture in the air is actually the big greenhouse gas. A small 1% rise in temperature from CO2 will cause more moisture to evaporate, which then raises the temperature more, which then causes more moisture to evaporate, which raises it more which causes more to evaporate .... etc.
It's like when you hold a microphone too close to a loudspeaker. As the speaker gets louder, the mic pics up more noise, making the speaker get louder.... until you hear a loud screech.
But fortunately the feedback effect of water moisture evaporation has a stopping point, because the increase temperature also causes more heat to radiate into space, but the Sun always adds the same amount. (Hotter objects radiate more heat than cooler objects.)
So we won't end up like Venus. But some bad things will surely happen.
a reply to: thepixelpusher
That's nice. Yes, increased CO2 levels can be the result of increased temperatures (due to ocean outgassing, mostly). But it's nonsense to say that means that higher CO2 levels can't cause increased temperatures. We know where the CO2 is coming from. It's not from the ocean. It is not the result of warming.
www.abovetopsecret.com...
But apart from that, what does it have to do with the claim you made:
The chart in your source seems to contradict your statement. It shows a peak of about 300 ppm (the green line), about 340 thousand years ago. Seems it's quite a bit higher than that now.
cdn2.collective-evolution.com...
That's nice. Yes, increased CO2 levels can be the result of increased temperatures (due to ocean outgassing, mostly). But it's nonsense to say that means that higher CO2 levels can't cause increased temperatures. We know where the CO2 is coming from. It's not from the ocean. It is not the result of warming.
www.abovetopsecret.com...
But apart from that, what does it have to do with the claim you made:
We have less CO2 in the air than the industrial era and ice cores prove that
The chart in your source seems to contradict your statement. It shows a peak of about 300 ppm (the green line), about 340 thousand years ago. Seems it's quite a bit higher than that now.
cdn2.collective-evolution.com...
edit on 11/27/2017 by Phage because: (no reason given)
originally posted by: thepixelpusher
a reply to: Phage
420,000 YEARS OF DATA SUGGESTS GLOBAL WARMING IS NOT ENTIRELY MAN-MADE
The Vostok ice core sample was obtained by drilling down into the ice above Lake Vostok to a depth of 3623m. The graph built from the Vostok ice core data shows us the relationship between CO2 in the atmosphere and global temperature. Contrary to current belief today, the Vostok data shows us that CO2 increases lag behind temperature increases by about 800 years. This means that CO2 is not the cause of the increased temperatures, although it might potentially play a small role. This cannot be confirmed at this time however. The Vostok graph also shows us the cyclical pattern that occurs with warming and cooling as well as the increase in CO2 levels.
The Vostok ice cores end around the year 1850, that's why CO2 is below 300 ppm.
Feel free to keep on embracing ignorance, but don't expect that it won't be called out on ATS.
edit on 15Mon, 27 Nov 2017 15:39:02
-0600America/ChicagovAmerica/Chicago11 by Greven because: (no reason given)
originally posted by: thepixelpusher
a reply to: Greven
The sun warms the earth, the sun goes through warming and cooling cycles. We have less CO2 in the air than the industrial era and ice cores prove that. Your argument is moot.
Uh, no, since Vostok CO2 is below 300 ppm.
It's been going up up up since...
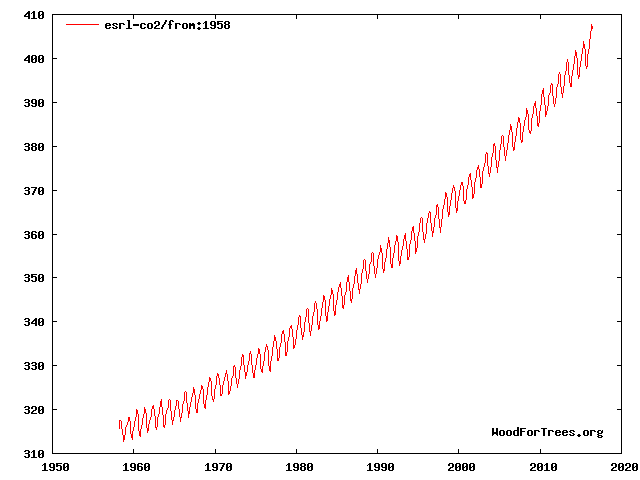
At the same time that oxygen levels are going down down down...
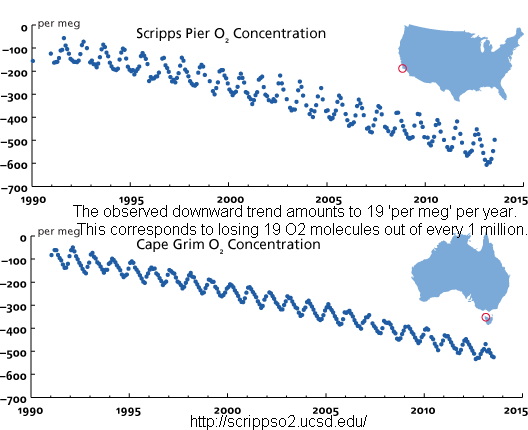
This would be due to us burning carbon which combines carbon with oxygen primarily from the atmosphere.
Oh, and like I said before, photosynthesis gets the oxygen part from water, not carbon dioxide, so it's not going to recover anytime soon.
edit on 15Mon, 27 Nov 2017 15:43:36 -0600America/ChicagovAmerica/Chicago11 by Greven because: (no reason given)
a reply to: Phage
a reply to: Greven
Did you two even read the article!? It has manmade CO2 at 3% of the total contribution. Your argument is moot! If you bothered to look at the charts this is cyclical...i.e. natural!!

a reply to: Greven
Did you two even read the article!? It has manmade CO2 at 3% of the total contribution. Your argument is moot! If you bothered to look at the charts this is cyclical...i.e. natural!!
Industry currently pumps 3% more CO2 into the atmosphere each year, which is only responsible for a total of .27% of the greenhouse effect. The reality this creates is that if we were to cease all transport and industry right now, it is very unrealistic to assume that it would have any impact on global warming. Since this cannot be stated as fact, we can leave this point open to possibility. However it is important to note that the claims made by major pushers of global warming greatly rely on the assumption that humanity’s small addition to the CO2 levels is what is going to push warming beyond a point of return. As you can see from the previous data, this assumption is not backed nor sound.

edit on 27-11-2017 by thepixelpusher because: (no reason given)
edit on 27-11-2017 by thepixelpusher
because: (no reason given)
a reply to: thepixelpusher
In reality, CO2 levels have increased by more than 40% since the beginning of the industrial era and are higher than they have been for at least 800,000 years. That increase is because of us.
rationalwiki.org...
Yeah. It does some interesting things with numbers (while ignoring feedback effects, see below for a bit about the author), but does it say that CO2 levels were higher before the industrial era?
Did you two even read the article!?
We have less CO2 in the air than the industrial era and ice cores prove that
In reality, CO2 levels have increased by more than 40% since the beginning of the industrial era and are higher than they have been for at least 800,000 years. That increase is because of us.
rationalwiki.org...
edit on 11/27/2017 by Phage because: (no reason given)
a reply to: thepixelpusher
I saw it. Actually. It's talking about all greenhouse gasses.
Yes, there are natural sources of CO2. However, those sources have not changed significantly since the industrial revolution. What has changed is the amount of CO2 which humans produce. We know the cause of that 40% increase in CO2. It is us, burning fossil fuels. Fossil fuels which contain no 14C.
www.abovetopsecret.com...
I saw it. Actually. It's talking about all greenhouse gasses.
Yes, there are natural sources of CO2. However, those sources have not changed significantly since the industrial revolution. What has changed is the amount of CO2 which humans produce. We know the cause of that 40% increase in CO2. It is us, burning fossil fuels. Fossil fuels which contain no 14C.
www.abovetopsecret.com...
edit on 11/27/2017 by Phage because: (no reason given)
originally posted by: mbkennel
originally posted by: M5xaz
originally posted by: mbkennel
originally posted by: M5xaz
originally posted by: DJW001
a reply to: liejunkie01
Principia-Scientia exists solely to publish anti climate change propaganda. Gases like CO2 exist in the form of molecules. These molecules bounce around at different speeds, mixing with other molecules in the atmosphere. The atmosphere is filled with carious current, including convection, analogous to the bubbles in boiling water. The paper cited in this hit piece ignores all that.
Wrong.
Basic fluid physics.
Higher density molecules like CO2 will necessarily concentrate in the lower portions of the atmosphere, while lighter gases rise.
Ah, that salty water business must be Fake Science too! As both sodium and chlorine have higher molecular weights than H2O, all of those elements must be lying on the bottom of the deepest ocean! Basic Physics! Ha!
Calling our American Oceans salty is an evil HOAX from Big Water and environazis and George Soros whose UN plot to preserving the supposed "fresh water" resources is a globalist attack on our precious bodily fluids!
Wrong again.
Dissolution of salt into ion in water is not the same as mixing different fluids.
What is the physics and chemistry of that difference which results in the observationally-false idea that CO2 is separated from the rest of the air?
Wrong yet again.
Heavier/denser gases accumulate in the lower atmosphere and lighter gases in the higher atmosphere.
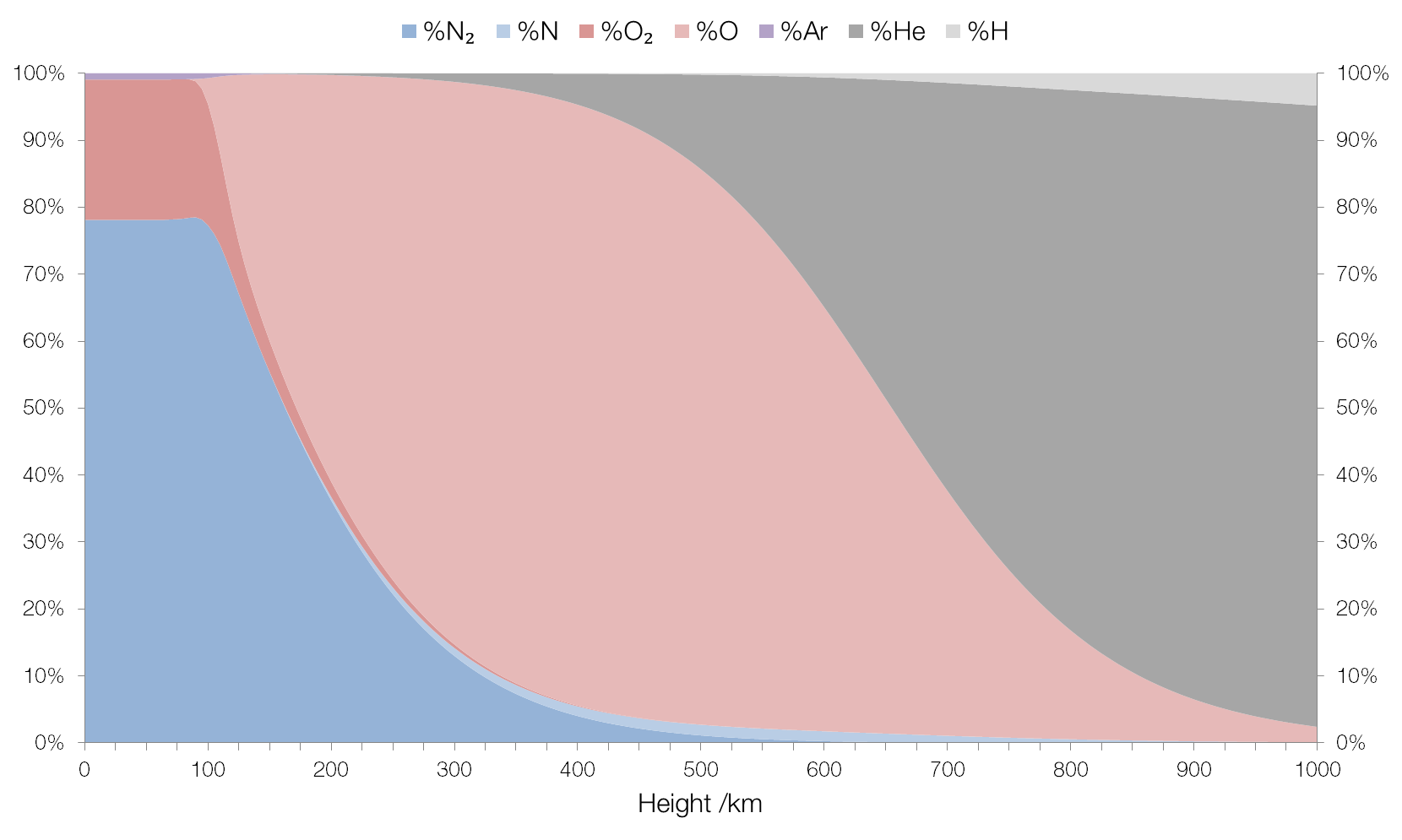
But don't let facts get in the way of your virtue-signalling "religion"
originally posted by: DJW001
a reply to: M5xaz
Actually, we do not really know what the Earth's atmosphere was like during its early history. Our models draw on elemental abundances combined with assumptions about insolation. Abundant light elements like hydrogen were likely boiled away if they were not bound up into heavier molecules. Venus serves as as a convenient model.
No, Venus does not.
Earth is at ZERO risk of becoming 95% CO2.
Present CO2 concentration is 400 PARTS PER MILLION.
Present Nitrogen concentration in the atmosphere is ~ 75%.
Nothing like Venus.
edit on 27-11-2017 by M5xaz because: (no reason given)
a reply to: liejunkie01
You do know that that site, is funded by Climate change deniers right? Just curious?
You do know that that site, is funded by Climate change deniers right? Just curious?
a reply to: M5xaz
That altitude scale is interesting. Your source:
wordpress.mrreid.org...
Are you familiar with something called the "Karman line?" There aren't really enough of any gasses to worry about at that altitude.
Concentrations of CO2 are a bit less in the higher parts of the atmosphere that matter. But not a great deal. At altitudes above 20km there is a decrease of about 5-6 ppm. It happens quite suddenly though. Probably because mixing is reduced at the tropopause.
www.atmos-chem-phys.net...
That altitude scale is interesting. Your source:
Up to around 100?km the composition is fairly “normal”, in that it’s what we surface-dwellers would expect: mostly molecular nitrogen (N2 rather than N) and molecular oxygen (O2) with a small amount (0.93%) of argon and traces of some other gases (carbon dioxide, neon, etc.).
wordpress.mrreid.org...
Are you familiar with something called the "Karman line?" There aren't really enough of any gasses to worry about at that altitude.
Concentrations of CO2 are a bit less in the higher parts of the atmosphere that matter. But not a great deal. At altitudes above 20km there is a decrease of about 5-6 ppm. It happens quite suddenly though. Probably because mixing is reduced at the tropopause.
www.atmos-chem-phys.net...
edit on 11/27/2017 by Phage because: (no reason given)
originally posted by: thepixelpusher
a reply to: Phage
a reply to: Greven
Did you two even read the article!? It has manmade CO2 at 3% of the total contribution. Your argument is moot! If you bothered to look at the charts this is cyclical...i.e. natural!!
Industry currently pumps 3% more CO2 into the atmosphere each year, which is only responsible for a total of .27% of the greenhouse effect. The reality this creates is that if we were to cease all transport and industry right now, it is very unrealistic to assume that it would have any impact on global warming. Since this cannot be stated as fact, we can leave this point open to possibility. However it is important to note that the claims made by major pushers of global warming greatly rely on the assumption that humanity’s small addition to the CO2 levels is what is going to push warming beyond a point of return. As you can see from the previous data, this assumption is not backed nor sound.
Sure, I read it. Perhaps you should read what you cite a bit better.
How many years do you have to pump 3% more CO2 into the atmosphere do you think it will take to be responsible for a majority of CO2 in the atmosphere?
The answer is less than 24 years. CO2 has not anywhere near doubled since preindustrial times.
Also, it's a bull# number, because we aren't putting that much CO2 into the atmosphere every year; it's about a 0.8% increase this year from last year.
Does this not tell you that the article is bull#?
How about this one:
It is believed that there is about 800 billion tonnes of CO2 in the atmosphere and human activities release about another 27 billion tonnes per year, or 3% of the total.
Wrong. I've already posted this once in the thread but might as well again...
originally posted by: Greven
...here's some math for you:
Earth's atmosphere: 5,148,000 gigatonnes (Gt) = a
Mean molar mass of the atmosphere: 28.97g/mole = b
Carbon Dioxide (CO2) molar mass: 44.0095 g/mole = c
Atmospheric CO2 parts per million (ppm), November 2014: 397.27 ppm = d
Atmospheric CO2 ppm, November 2015: 400.16 ppm = e
Atmospheric CO2 mass, November 2014 (a * (c / b) * d): 3,106.7812 Gt = f
Atmospheric CO2 mass, November 2015 (a * (c / b) * e): 3,129.4654 Gt = g
Atmospheric CO2 mass increase (g - f): 22.6842 Gt
That's only a partial representation of humanity's estimated emissions for the year, since the biosphere is still acting as a net sink.
...
So, let's look at historical data
Atmospheric CO2 parts per million, 2000 mean: 368.80 ppm = h
Atmospheric CO2 parts per million, 2010 mean: 388.58 ppm = i
Atmospheric CO2 mass, November 2000 (a * (c / b) * h): 2884.2134 Gt = j
Atmospheric CO2 mass, November 2010 (a * (c / b) * i): 3038.9036 Gt = k
Atmospheric CO2 mass increase (k - j): 154.6902 Gt
An increase of 15.46902 Gt/yr (2000-2010). Compare that with the 22.6842 Gt/yr increase from 2014-2015.
If atmospheric CO2 was only 800 billion tonnes (billion tonne = gigatonne), then CO2 would comprise only 102 ppm. That's not just wrong, it's also stupid.
Please stop citing idiotic articles.
a reply to: M5xaz
Reading comprehension issues. No-one has said that CO2 will eventually displace nitrogen as the main component of our atmosphere. When we look at worlds like Titan, we see that organic molecules are found in their atmosphere, further evidence that early Earth had a great deal of CO2. Photosynthesis has reduced the amount of carbon dioxide in the atmosphere, but human activity has been reducing the amount of plant life sequestering CO2 while releasing more by burning "fossil fuels." Models suggest that even a small amount of increase can lead to global temperatures rising. Earth does not care, it is humanity that might suffer as sea levels rise and storms get more intense.
Reading comprehension issues. No-one has said that CO2 will eventually displace nitrogen as the main component of our atmosphere. When we look at worlds like Titan, we see that organic molecules are found in their atmosphere, further evidence that early Earth had a great deal of CO2. Photosynthesis has reduced the amount of carbon dioxide in the atmosphere, but human activity has been reducing the amount of plant life sequestering CO2 while releasing more by burning "fossil fuels." Models suggest that even a small amount of increase can lead to global temperatures rising. Earth does not care, it is humanity that might suffer as sea levels rise and storms get more intense.
edit on 27-11-2017 by DJW001 because: (no
reason given)
originally posted by: M5xaz
originally posted by: mbkennel
originally posted by: M5xaz
originally posted by: mbkennel
originally posted by: M5xaz
originally posted by: DJW001
a reply to: liejunkie01
Principia-Scientia exists solely to publish anti climate change propaganda. Gases like CO2 exist in the form of molecules. These molecules bounce around at different speeds, mixing with other molecules in the atmosphere. The atmosphere is filled with carious current, including convection, analogous to the bubbles in boiling water. The paper cited in this hit piece ignores all that.
Wrong.
Basic fluid physics.
Higher density molecules like CO2 will necessarily concentrate in the lower portions of the atmosphere, while lighter gases rise.
Ah, that salty water business must be Fake Science too! As both sodium and chlorine have higher molecular weights than H2O, all of those elements must be lying on the bottom of the deepest ocean! Basic Physics! Ha!
Calling our American Oceans salty is an evil HOAX from Big Water and environazis and George Soros whose UN plot to preserving the supposed "fresh water" resources is a globalist attack on our precious bodily fluids!
Wrong again.
Dissolution of salt into ion in water is not the same as mixing different fluids.
What is the physics and chemistry of that difference which results in the observationally-false idea that CO2 is separated from the rest of the air?
Wrong yet again.
Heavier/denser gases accumulate in the lower atmosphere and lighter gases in the higher atmosphere.
But don't let facts get in the way of your virtue-signalling "religion"
Uh, it seems you are unaware that 77.7% of all atmospheric mass is within the first 11km above the surface, and that an additional 18.1% of all atmospheric mass is within 11km to 20km above the surface. A further 3% is within the 20km to 32km range. That would be 99% by this point... any molecules above 32km comprises 1% of atmospheric mass combined, with 0.9% of that 1% being below 50km.
Notice that your chart uses 100km markers and 25km ticks... and realize that virtually all atmospheric mass is within the very first tick above 0.
I mean seriously, the space shuttle orbited Earth up to 530km out... that's halfway across this chart that you've grossly misused. The International Space Station orbits at about 400km. Talk about not understanding reality.
edit on 18Mon, 27 Nov 2017 18:18:22
-0600America/ChicagovAmerica/Chicago11 by Greven because: (no reason given)
a reply to: M5xaz
They pretty much are, actually. Until you reach "space." Up there chemistry and radiation start doing things. Which is exactly what you source says:
wordpress.mrreid.org...
Merely responding to the uninformed posters claiming heavier gases are uniformly mixed in the atmosphere.
They pretty much are, actually. Until you reach "space." Up there chemistry and radiation start doing things. Which is exactly what you source says:
Up to around 100?km the composition is fairly “normal”, in that it’s what we surface-dwellers would expect: mostly molecular nitrogen (N2 rather than N) and molecular oxygen (O2) with a small amount (0.93%) of argon and traces of some other gases (carbon dioxide, neon, etc.).
wordpress.mrreid.org...
edit on 11/27/2017 by Phage because: (no reason given)
a reply to: M5xaz
Not really, not enough to matter.
www.atmos-chem-phys.net...
Heavier/denser gases accumulate in the lower atmosphere and lighter gases in the higher atmosphere.
Not really, not enough to matter.
www.atmos-chem-phys.net...
originally posted by: Greven
originally posted by: bronco73
originally posted by: Greven
Principa-Scientific, the source for this utter nonsense, is a ridiculous shill site that just makes up stuff to go with its ideological leanings.
Honestly, it should be banned from linking like the rest of the ones that are, for the same reasons.
CO2 measurements are taken on top of a volcano in Hawaii, several thousand feet up down to sea level in other locations. There are hundreds of stations that record CO2 all over the world at varying altitudes, yet the variation is not enormous. The most variation is in Antarctica, as I recall.
Yes, CO2 is heavier than O2 and N2. No, it does not all fall down to the surface and cluster at ground level, because we would all have suffocated long ago if it did.
wow man..... VEGETATION?
Vegetation also needs oxygen, so it too would be dead. While the green bits get oxygen from photosynthesis (specifically, the splitting of water into H2 and O, as the CO2 part is used for carbohydrates), the roots need oxygen from the air. That's why you can drown plants in water. Some plants have evolved to live in wet conditions, either tolerating the lower oxygen content in water or (like with mangroves) alternative means of getting oxygen to the roots.
If CO2 clustered near the surface like that idiotic article and several people seem to believe in this thread, then there would be no life on this rock.
True but you are thinking backwards. The reason we don't have and can't have 100% CO2 on the surface of the Earth is because vegetation has existed for at least as long as fauna, performing photosynthesis during the entire time. The accusation I replied to was that on Earth if CO2 settles to the surface we would all have suffocated long ago. Flora provides at least a large part of the reason why we would not.
new topics
-
Slow moving ufo over Mexico volcano Popocatepetl 8 May 2024
Aliens and UFOs: 3 hours ago -
A new Why Files How CRISPR and AI Destroy the World
Science & Technology: 4 hours ago -
Ask AI Is A Hot Mess
Links & Other Resources: 4 hours ago -
Battle of the Bay 2024 ; Tampa, Florida Special Forces Demonstration
Military Projects: 9 hours ago -
Tschugger
Movies: 10 hours ago -
Christianity superior to other faiths for very specific reasons. Awaken to true FREEDOM..!!
Conspiracies in Religions: 11 hours ago
top topics
-
The biggest problem with the Hush money trial
US Political Madness: 17 hours ago, 8 flags -
Christianity superior to other faiths for very specific reasons. Awaken to true FREEDOM..!!
Conspiracies in Religions: 11 hours ago, 7 flags -
A new Why Files How CRISPR and AI Destroy the World
Science & Technology: 4 hours ago, 5 flags -
It's all Kicking off at Eurovision 2024
Music: 16 hours ago, 4 flags -
Ask AI Is A Hot Mess
Links & Other Resources: 4 hours ago, 4 flags -
Tschugger
Movies: 10 hours ago, 3 flags -
Battle of the Bay 2024 ; Tampa, Florida Special Forces Demonstration
Military Projects: 9 hours ago, 3 flags -
Slow moving ufo over Mexico volcano Popocatepetl 8 May 2024
Aliens and UFOs: 3 hours ago, 2 flags
active topics
-
Barron Trump has prior commitments?
Politicians & People • 102 • : BingoMcGoof -
Bibi’s Dilemma
Middle East Issues • 203 • : AliceTheSmall -
Ooooh...it worked!!
Members • 29 • : toadaly -
Ask AI Is A Hot Mess
Links & Other Resources • 14 • : kwaka -
'You need help': Ashli Babbitt's mom fumes after MAGA rioter suggests her death was faked
Political Conspiracies • 83 • : 777Vader -
A new Why Files How CRISPR and AI Destroy the World
Science & Technology • 4 • : charlest2 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 948 • : brewtiger123 -
Scientists Find 7 potential Dyson spheres after Scanning 5 million Objects
Space Exploration • 39 • : toadaly -
Doctors Predict Epidemic of Prion Brain Diseases From mRna Jab
Health & Wellness • 73 • : chr0naut -
Christianity superior to other faiths for very specific reasons. Awaken to true FREEDOM..!!
Conspiracies in Religions • 20 • : jofafot
