It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: TiredofControlFreaks
a reply to: mbkennel
oh so we can't "save the planet" until 7 billion people either freeze or boil themselves and do without cooked food for a year.
Good luck with that
That's why it's imperative to start to change the technology and the economy as early as possible, which was about 1993. And global economic incentives are the most efficient way to do so, as has been shown by the successful anti-acid rain policies since the late 1980's.
a reply to: mbkennel
I would like to comment on your tiny little statement about "global incentives"
You do understand that the reason why COP17 had an agreement to pay underdeveloped countries, what was it, 100 billion or so, from developed countries?
We are actually paying them not to develop. People in Africa must continue to live in huts so that there will little downgrading of the use of fossil fuels in developed countries.
This is what you call the most 'effective" solution?
Tired of Control Freaks
I would like to comment on your tiny little statement about "global incentives"
You do understand that the reason why COP17 had an agreement to pay underdeveloped countries, what was it, 100 billion or so, from developed countries?
We are actually paying them not to develop. People in Africa must continue to live in huts so that there will little downgrading of the use of fossil fuels in developed countries.
This is what you call the most 'effective" solution?
Tired of Control Freaks
a reply to: TiredofControlFreaks
I have been looking at charts of Arctic Sea Ice and I do have a couple of comments
nsidc.org...
First of all - the grey area represents the average sea ice from 1981 to 2010 with a standard deviation of plus or minus 2
As far as I remember from my college stats course - that represents variability within the norm.
Now its easy to understand that the global temperature was down from about 1945 to the late 1970s - so including 1981 skews comparison to the norm because it includes ice from a time when global temeperature anomalies were in the negative.
however, given the scarceness of date from 1970s, I won't quibble but I think we need to remember that it might skew the norm toward showing more loss than we should be expecting.
Second - The spring is when the most sea ice is present and the fall is when the least sea ice is present.
I have found that, in general, the curves for every year pretty well stay within the norm. An exception would be 2014 but since the oceans were running hot prior to El Nino, I don't consider that much to worry about. It appears to me that 2013 is pretty much the same as 1998.
I don't see what everyone is on about the Arctic is a harbringer of doom!
Further, I think the data includes Hudson's Bay. I don't think Hudson's Bay is part of the Arctic Ocean. Because Hudson's Bay has far lower salinity than an ocean.
Tired of Control Freaks
I have been looking at charts of Arctic Sea Ice and I do have a couple of comments
nsidc.org...
First of all - the grey area represents the average sea ice from 1981 to 2010 with a standard deviation of plus or minus 2
As far as I remember from my college stats course - that represents variability within the norm.
Now its easy to understand that the global temperature was down from about 1945 to the late 1970s - so including 1981 skews comparison to the norm because it includes ice from a time when global temeperature anomalies were in the negative.
however, given the scarceness of date from 1970s, I won't quibble but I think we need to remember that it might skew the norm toward showing more loss than we should be expecting.
Second - The spring is when the most sea ice is present and the fall is when the least sea ice is present.
I have found that, in general, the curves for every year pretty well stay within the norm. An exception would be 2014 but since the oceans were running hot prior to El Nino, I don't consider that much to worry about. It appears to me that 2013 is pretty much the same as 1998.
I don't see what everyone is on about the Arctic is a harbringer of doom!
Further, I think the data includes Hudson's Bay. I don't think Hudson's Bay is part of the Arctic Ocean. Because Hudson's Bay has far lower salinity than an ocean.
Tired of Control Freaks
In the GTA in Canada, the winter was a mild one this year and yet we still had ass biting wind chill, not the first time though we didn't have any
snow but it still rained. It hasn't rain much other then monumental flash showers, and only had a few thunderstorms, and there are large patches of
bash grass almost every where. We even almost had freezing rain that almost encased everything in ice again this year, but luck would have it, it
thawed. Something similar happened a 2 or 3 years ago, but everything was in ice for a week on X-mas holiday week 2013, I think it was around the same
that solar flare happened. The previous generation of the area never saw anything like it before.
It was beautiful to see the trees like it but it was deadly.
IMO, I'd say both people, and natural occurrences are the cause, due to how imbalanced the ecosystem has been, but all the while the climate has it ups and downs of hot an cold. I think the CO2 would be more of temporary problem if we could just shut down the National Machines for a while and let the air cool filter out, but would not be a remedy for ice that been around for thousands of years can do.
It was beautiful to see the trees like it but it was deadly.
IMO, I'd say both people, and natural occurrences are the cause, due to how imbalanced the ecosystem has been, but all the while the climate has it ups and downs of hot an cold. I think the CO2 would be more of temporary problem if we could just shut down the National Machines for a while and let the air cool filter out, but would not be a remedy for ice that been around for thousands of years can do.
edit on 22-7-2016 by Specimen because: (no reason given)
edit on 22-7-2016 by Specimen because: (no reason given)
originally posted by: Nathan-D
First off, they're not observations. Screwing around with data is altering the observations. Secondly, while atmospheric CO2 is increasing, dissolved CO2 is increasing at a similar pace. If it's supposed to be responsible for the increase in CO2, please explain how.
I should point out that this was data collected from one station in Hawaii in 1973. That was 43 years ago. Anyway, the ocean is a big place and that one station probably represents 0.0001% of the entire ocean. Unless we are willing and able to cover the ocean’s entire surface in a rather fine-meshed network our readings of PCO2 from only one station are liable to be too inaccurate to be useful and to be misleading accordingly. Furthermore the Hawaii ALOHA station is located in relatively shallow coastal waters and it has been shown that biological activity in coastal waters can significantly affect the the concentration of dissolved PCO2 (Evans et al 2011). Has this been considered?
Oh no, it was a long time ago. I think mbkennel dealt with this sufficiently.
originally posted by: Nathan-D
We have estimates of how much carbon we emit. Note that this is in carbon, not carbon dioxide. Transform that data into CO2 to calculate changes; O=15.9994 C=12.0107; CO2 is 27.2912 % carbon by mass, so (for example) 9 gigatonnes of carbon emissions ~ 33 gigatonnes of CO2 emissions. We can transform that as well:
Earth's atmosphere: 5,148,000 gigatonnes (Gt) = a
Mean molar mass of the atmosphere: 28.97g/mole = b
Carbon Dioxide (CO2) molar mass: 44.0095 g/mole = c
Atmospheric CO2 parts per million (ppm), June 2016: 406.81 ppm = d
Atmospheric CO2 ppm, June 2015: 402.80 ppm = e
Atmospheric CO2 mass, June 2015 (a * (c / b) * d): 3,150.1116 Gt = f
Atmospheric CO2 mass, June 2016 (a * (c / b) * e): 3,181.4719 Gt = g
Atmospheric CO2 mass increase (g - f): 31.3603 Gt
Your figure of 31Gts is merely the increase in atmospheric CO2 from 2015 to 2016. That does not mean that the human contribution of 33Gts is the cause of the increase. You have to understand Henry’s law in order to understand why. In order to explain how human CO2 is accumulating in the atmosphere the IPCC apply the Revelle Factor which contradicts Henry’s law. Henry’s law determines a specific fixed ‘partitioning ratio’ between the amount of CO2 residing in the atmosphere and the amount that will be dissolved in the oceans at a given temperature at equilibrium. At the current mean ocean temperature of ~15°C (at the surface), that partitioning ratio comes out to be ~1:50. This implies that if humans added 33Gts of CO2 to the atmosphere/year the amount remaining in the atmosphere upon equilibrium would be 33/50 = 0.66Gts/year. This is equivalent to about 0.085ppmv/year. Now the partitioning ratio is temperature-dependent. So if the oceans were to warm to say ~20°C (a 5°C temperature increase), the partitioning ratio would rise to ~1:40 and CO2 would be released from the oceans to be permanently added to the resident CO2 greenhouse.
Here is a thought-experiment to illustrate the point: Say we had a can of soda-pop with an average temperature of 15°C and added 100 grams of CO2 into the head-space above the water. The CO2 would rapidly equilibrate with the water until 98 grams has been dissolved and 2 grams resided in the head-space in accordance with the 1:50 partitioning ratio at that temperature. Now imagine at the same time we increased the water-temperature by 5°C and thereupon shifted the partitioning ratio to 1:40. Instead of 0.98 grams being dissolved into the water 97.5 grams would be dissolved, leaving 2.5 grams in the head-space. At the same time, some CO2 would be released from the water due to the temperature-change, let’s assume 10 grams. The end result is that the water has simultaneously absorbed 97.5% (essentially all) of the CO2 we added while increasing the CO2 in the head-space due to the temperature-change. This is why the mass-balance argument (as it is being inappropriately applied by warmists) is invalid.
How about this then:
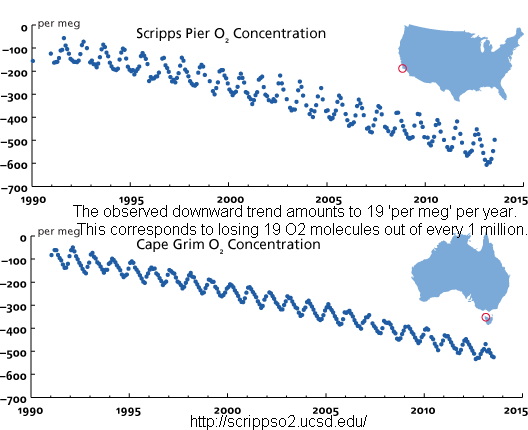
It's pretty damn simple - our carbon emissions are primarily burnt, creating a whole lot of carbon dioxide out of that emitted carbon and the oxygen in the atmosphere. Since that oxygen has to come from somewhere, we are lowering the oxygen in the atmosphere. How well does that fit with your ideas about Henry’s law?
Oh, and here's another quick fact: photosynthesis does not turn CO2 into air that we breathe. Read that again - twice if you have to. Yes, photosynthesis produces O2, but that O2 is from (multiple)H2O.
What do you think the end game is, based on what we are seeing - an increase in CO2 and a decrease in O2?
originally posted by: TiredofControlFreaks
a reply to: Greven
I believe that it has much more to do with the oceans than with any part of the atmosphere. Remember that the oceans comprise something over 70 % of the earth. A far greater part than land.
I think we need to really understand ocean currents if we want to understand climate.
Particulary, the PDO occurring in the largest ocean and the AMO occurring the atlantic.
For the record - with the ending of El Nino - both are cooling at the same time. What will develop from this cannot be atmospheric warming.
I think we are seeing the last great hurrah for AGW.
A lot of people believe in a lot of different things, but that doesn't make any of them right.
Worse, your 'counter' here is completely meaningless. You fail to address the points entirely. Instead you handwave away the argument, saying 'the oceans' as if that makes the Earth disobey the laws of physics.
Again:
Explain how Earth's surface is so much warmer than it ought to be and why it's cooler in the troposphere as you go up.
originally posted by: TiredofControlFreaks
a reply to: jrod
It takes even more to suggest that CO2 rises are from anthropogenic sources while use of fossil fuels has remained flat since 2013.
Uh... no?
If the amount of water being put into drain is already overflowing, keeping that amount steady doesn't mean the water is going to go down.
Keeping our excessive CO2 emissions the same isn't going to decrease the CO2 in the atmosphere.
originally posted by: Specimen
In the GTA in Canada, the winter was a mild one this year and yet we still had ass biting wind chill, not the first time though we didn't have any snow but it still rained. It hasn't rain much other then monumental flash showers, and only had a few thunderstorms, and there are large patches of bash grass almost every where. We even almost had freezing rain that almost encased everything in ice again this year, but luck would have it, it thawed. Something similar happened a 2 or 3 years ago, but everything was in ice for a week on X-mas holiday week 2013, I think it was around the same that solar flare happened. The previous generation of the area never saw anything like it before.
It was beautiful to see the trees like it but it was deadly.
IMO, I'd say both people, and natural occurrences are the cause, due to how imbalanced the ecosystem has been, but all the while the climate has it ups and downs of hot an cold. I think the CO2 would be more of temporary problem if we could just shut down the National Machines for a while and let the air cool filter out, but would not be a remedy for ice that been around for thousands of years can do.
How is CO2 'filtered out?'
It's pretty damn simple - our carbon emissions are primarily burnt, creating a whole lot of carbon dioxide out of that emitted carbon and the oxygen in the atmosphere. Since that oxygen has to come from somewhere, we are lowering the oxygen in the atmosphere. How well does that fit with your ideas about Henry’s law?
Sure, O2 decreases when anthropogenic CO2 binds to it, but I think any isotopic argument used to try and prove that humans have contributed 40% to the CO2 increase is misleading because anthropogenic CO2 is absorbed rapidly. By the IPCC’s own figures the ratio of anthropogenic CO2 emissions to naturogenic ones is approx. 4%:96% respectively, meaning that the anthropogenic emissions constitute 4% of the total. These proportions are retained throughout the cycle of emission and absorption and also apply to the residual left over from the cycle which could not be re-absorbed into sinks and which remains in the atmosphere as a permanent increment to the atmospheric greenhouse. It follows then that the CO2 present in the atmosphere at any time is (at most) about 4% anthropogenic. The same considerations apply to other isotopic changes, such as the decrease in C14:
A common argument that the atmospheric CO2 increase is anthropogenic is the observed decrease in 14CO2 in the atmosphere. Anthropogenic CO2 is depleted in 14C and so increasing it will lead to decreased 14CO2 levels. However the fact that 14CO2 is decreasing is not proof that the increase in CO2 is anthropogenic because 14CO2 in the atmosphere would be expected to decrease simply by virtue of our emissions increasing and the atmospheric CO2 mass increasing. As proof of this last statement I offer the following argument which I have already rehearsed above and so will just repeat it briefly here for now. It goes as follows: From IPCC’s AR5 (2013) we have: Human CO2 emissions = 33Gts/year, natural CO2 emissions = 724Gt/year, and natural absorption = 745Gts/year. Meanwhile the atmospheric CO2 mass stands at about 3,120 (400ppmv) which gives us a residence time of 3,120/745 = 4.2 years. This means the total amount of anthropogenic CO2 residing in the atmosphere before absorption is 33*4.2 = 138.6Gts = 17.8ppmv. Assuming that the atmospheric CO2 mass is increasing at the rate of 2ppmv/year (15.6Gts) then by the end of 5 years that would have increased the residence time to 3198/745 = 4.3 years. Assuming also that our emissions increase from 33Gts to 36Gts then the amount of anthropogenic CO2 residing in the atmosphere before absorption would be 36*4.3 = 154.8Gts = 19.8ppmv (which is of course higher than 17.8ppmv). This illustrates that 14CO2 levels decreasing in the atmosphere is simply a consequence of the atmospheric CO2 mass increasing (which increases residence time) together with our emissions increasing, and is not ‘proof’ that humans are driving the increase.
How well does that fit with your ideas about Henry’s law?
My ideas? You mean the idea that water releases CO2 when warmed or the idea that to maintain equilibrium water absorbs CO2 when you increase the partial pressure in accordance with Le Chatelier’s principle? Those are not so much ‘my ideas’, as they are well-established tenets of orthodox science. To calculate an increase in CO2 from a change in water-temperature you can just apply the Van’t-Hoff equation, and while my calculations differ from Jaworowski’s, it can still be shown that some of the increase in atmospheric CO2 would have come from ocean warming. Humans are not entirely responsible. That’s the point.
Oh, and here's another quick fact: photosynthesis does not turn CO2 into air that we breathe. Read that again - twice if you have to. Yes, photosynthesis produces O2, but that O2 is from (multiple) H2O.
Not sure what you‘re getting at here or why you‘re bringing this up.
I think mbkennel dealt with this sufficiently.
With respect, I don’t think my question has been ‘sufficiently dealt with’. He posted a map showing Argo floats and I asked for a graph showing decreased PCO2(aq) from those 3,000 floats. The only graph I have seen of decreased concentrations of PCO2(aq) is the Hawaii time-series.
edit
on 22-7-2016 by Nathan-D because: (no reason given)
originally posted by: TiredofControlFreaks
a reply to: Greven
greven
Thank you for proving that burning fossil fuels is the source of CO2 in the atmosphere. But I don't believe that is the issue. The issue is "how sensitive is the climate to the CO2 forcing".
Tired of Control Freaks
I agree. The most important issue is CO2 radiative forcing. I’ve already accepted (on the last page) that the majority of the increase in atmospheric CO2 is human-induced, and yet somehow we’re still arguing about it.
a reply to: Astyanax
There is no doubt that the HAARP apparatus is being used to change the weather.
The official patent for HAARP is online. Go to it and click on find "weather modification" and you can see for yourself.
patft.uspto.gov.../netahtml/PTO/srchnum.htm&r=1&f=G&l=50&s1=4,686,605.PN.&OS=PN/4,686,605 &RS=PN/4,686,605
Anyone remember the ex-President of Iran complaining about it? Read about it here:
www.telegraph.co.uk...
EDIT: Interesting that when I posted the link to HAARP patent, it breaks the link. Trying copying these two halves of it and putting them together:
patft.uspto.gov...
netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=/netahtml/PTO/srchnum.htm&r=1&f=G&l=50&s1=4,686,605.PN.&OS=PN/4,686,605&RS=PN/4,686,605
There is no doubt that the HAARP apparatus is being used to change the weather.
The official patent for HAARP is online. Go to it and click on find "weather modification" and you can see for yourself.
patft.uspto.gov.../netahtml/PTO/srchnum.htm&r=1&f=G&l=50&s1=4,686,605.PN.&OS=PN/4,686,605 &RS=PN/4,686,605
Anyone remember the ex-President of Iran complaining about it? Read about it here:
www.telegraph.co.uk...
EDIT: Interesting that when I posted the link to HAARP patent, it breaks the link. Trying copying these two halves of it and putting them together:
patft.uspto.gov...
netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&d=PALL&p=1&u=/netahtml/PTO/srchnum.htm&r=1&f=G&l=50&s1=4,686,605.PN.&OS=PN/4,686,605&RS=PN/4,686,605
edit on 22-7-2016 by rottensociety because: broken link
originally posted by: TiredofControlFreaks
a reply to: mbkennel
Why ever do you think the acid rain issue was resolved?
Tired of Control Freaks
Because it was, through control of sulfur emissions from power plants, effectively incentivized in an economically efficient way by a cap and trade agreement.
en.wikipedia.org...
originally posted by: Nathan-D
[
My ideas? You mean the idea that water releases CO2 when warmed or the idea that to maintain equilibrium water absorbs CO2 when you increase the partial pressure in accordance with Le Chatelier’s principle? Those are not so much ‘my ideas’, as they are well-established tenets of orthodox science. To calculate an increase in CO2 from a change in water-temperature you can just apply the Van’t-Hoff equation, and while my calculations differ from Jaworowski’s, it can still be shown that some of the increase in atmospheric CO2 would have come from ocean warming. Humans are not entirely responsible. That’s the point.
That would be interesting if oceans were getting less acidic and carbon is being removed from the ocean and deposited into the atmosphere, but that is the opposite of observed fact. Of course there is a turnover of man-emitted and pre-human atmospheric carbon to and from the ocean & atmosphere.
With respect, I don’t think my question has been ‘sufficiently dealt with’. He posted a map showing Argo floats and I asked for a graph showing decreased PCO2(aq) from those 3,000 floats. The only graph I have seen of decreased concentrations of PCO2(aq) is the Hawaii time-series.
Do you have any idea about the depth of the professional activity in the field? I'm far from an insider but just a little bit of googling can get you far.
www.oco.noaa.gov...
Just one publication. I don't know the details of what really went into this but the idea that oceans are acidifying as a commonly understood observational fact is not based on one observation from Hawaii. Really, do you believe professional scientists are that naive?
cdiac.ornl.gov...
Climatological Distributions of pH, pCO2, Total CO2, Alkalinity, and CaCO3 Saturation in the Global Surface Ocean
by Taro Takahashi,1, Stewart C. Sutherland1, David W. Chipman1, John G. Goddard1, Timothy Newberger2 and Colm Sweeney2
Prepared by Alex Kozyr3
1Lamont-Doherty Earth Observatory of Columbia University, Palisades, NY 10964, USA
2Cooperative Institute in Environmental Sciences, University of Colorado, Boulder, CO 80309, USA
3Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, Tennessee, USA
dataNDP-094 Database Data and Maps PDF file NDP-094 (PDF format) ODV collection of the database
image
Abstract
Climatological mean monthly distributions of pH in the total H+ scale, total CO2 concentration (TCO2), and the degree of CaCO3 saturation for the global surface ocean waters (excluding coastal areas) are calculated using a data set for pCO2, alkalinity and nutrient concentrations in surface waters (depths less than 50 m), which is built upon the GLODAP, CARINA and LDEO database. The mutual consistency among these measured parameters is demonstrated using the inorganic carbon chemistry model with the dissociation constants for carbonic acid by Lueker et al. (2000) and for boric acid by Dickson (1990). The global ocean is divided into 24 regions, and the linear potential alkalinity (total alkalinity + nitrate) versus salinity relationships are established for each region. The mean monthly distributions of pH and carbon chemistry parameters for the reference year 2005 are computed using the climatological mean monthly pCO2 data adjusted to a reference year 2005 and the alkalinity estimated from the potential alkalinity versus salinity relationships. The climatological monthly mean values of pCO2 over the global ocean are compiled for a 4° x 5° grid for the reference year 2005, and the gridded data for each of 12 months are included in this database. This is updated version of Takahashi et al. (2009) for the reference year 2000 representing non-El Niño years using a database of about 6.5 million pCO2 data (less coastal areas of North and South America) observed in 1957-2012 (Takahashi et al., 2013). The equatorial zone (4°N-4°S) of the Pacific is excluded from the analysis because of the large interannual changes associated with the El Niño-Southern Oscillation events. The pH thus calculated ranges from 7.9 to 8.2. Lower values are located in the upwelling regions in the tropical Pacific and in the Arabian and Bering Seas; and higher values are found in the subpolar and polar waters during the spring-summer months of intense photosynthetic production. The vast areas of subtropical oceans have seasonally varying pH values ranging from 8.05 during warmer months to 8.15 during colder months. The warm tropical and subtropical waters are supersaturated by a factor of as much as 4.2 with respect to aragonite and 6.3 for calcite, whereas the cold subpolar and polar waters are less supersaturated only by 1.2 for aragonite and 2 for calcite because of the lower pH values resulting from greater TCO2 concentrations. In the western Arctic Ocean, aragonite undersaturation is observed.
www.oco.noaa.gov...
edit on 23-7-2016 by mbkennel because: (no reason given)
a reply to: Astyanax
I don't think too many are denying the existence of climate change, nor that humans are the primary cause of the increase in CO2 concentration in the atmosphere. The only debate lies in how much of an impact this increased CO2 concentration has on climate change. After 27 pages, as expected, I doubt that debate will be settled.
Here's my personal observation/experience on the subject:
The max daily temperature where I live has generally increased throughout my lifetime and is increasing more rapidly as time goes by.
The rainfall is changing. We experience more days of heavy rain but less days of light drizzle now.
The sun feels hotter and seems more penetrating now than when I was a kid.
From this, I concur that local warming is real and imagine that if it's happening where I am, it's likely to be happening elsewhere, so have no reason to dispute global warming/climate change.
Beyond this, I must rely on information supplied by others. From that information, there seems to be an increase in CO2 concentration in the atmosphere that coincides with increased burning of fossil fuels. The link to humans seems pretty solid, but in no way suggests that humans are primarily responsible for climate change. Much more investigation is needed to prove that an increase of CO2 in the atmosphere from 0.028% to 0.04% is the overwhelming driver behind climate change, and therefore a reduction in CO2 will fix the problem.
Now I'm not sure how the peer-reviewed system works, but unless anyone is able to publish a paper and have it peer-reviewed, then the integrity of this process must be analysed more closely before blindly accepting anything peer reviewed as Gospel. If a pre-requesite to publishing is membership, money, qualifications, contacts, support, etc then there is a possibility that the data pool is biased. If there is some sort of selection process involved before publishing, then there is a possibility that not all studies have been granted equal treatment. One needs to know the answers to these questions before accepting the validity of the argument that 98% of scientists are in agreement on the cause of climate change.
I've often been confounded by how they come up with a global temperature. There just seems to be so many variables. Are they talking about maximum temps, minimum temps, averages of temps taken every minute, hour, day or week? Are they using the same methods now as they were in the beginning and throughout the lifecycle of the data collectiojn period? Are they taking them equally over land and sea? Are they taken equidistant from eachother both horizontally and vertically?
Obviously there is much more to say on the subject of integrity but I think you get my point. At the end of the day, the best way to prove if climate change is our fault, would be to determine if it's happening on the other planets in the solar system as well. Can we reliably determine this? I don't know.
As far as doing something about climate change. Well, you may say it doesn't matter who is to blame, but that is quite a significant point. If we are to blame, then we can definitely do something about it and should be acting immediately. However, if we are not to blame, then it is futile to think that we can do anything to stop it. Pretty important point, don't you think? The good news is that we can actually do a lot to reduce the burning of fossil fuels without negatively impacting on the population significantly, just in case it is all our fault. There is already ample evidence out there to suggest that fossil fuel burning is having a detrimental impact on our planet in non-climate change ways. So a move away from fossil fuels is to be encouraged, especially when we have this huge source of free energy called the Sun, just sitting there each day. I just don't think imposing a huge tax on carbon usage is a smart move, when so many alternatives exist that can produce the same outcome, such as encouraging innovation and exploration in new technologies that favour renewable energy over fossil fuels.
Now we can pretend, that we don't know what the answers are, but the reality is, none of the answers either make enough money or preserve enough control for those in power to make it worthwhile implementing.
I don't think too many are denying the existence of climate change, nor that humans are the primary cause of the increase in CO2 concentration in the atmosphere. The only debate lies in how much of an impact this increased CO2 concentration has on climate change. After 27 pages, as expected, I doubt that debate will be settled.
Here's my personal observation/experience on the subject:
The max daily temperature where I live has generally increased throughout my lifetime and is increasing more rapidly as time goes by.
The rainfall is changing. We experience more days of heavy rain but less days of light drizzle now.
The sun feels hotter and seems more penetrating now than when I was a kid.
From this, I concur that local warming is real and imagine that if it's happening where I am, it's likely to be happening elsewhere, so have no reason to dispute global warming/climate change.
Beyond this, I must rely on information supplied by others. From that information, there seems to be an increase in CO2 concentration in the atmosphere that coincides with increased burning of fossil fuels. The link to humans seems pretty solid, but in no way suggests that humans are primarily responsible for climate change. Much more investigation is needed to prove that an increase of CO2 in the atmosphere from 0.028% to 0.04% is the overwhelming driver behind climate change, and therefore a reduction in CO2 will fix the problem.
Now I'm not sure how the peer-reviewed system works, but unless anyone is able to publish a paper and have it peer-reviewed, then the integrity of this process must be analysed more closely before blindly accepting anything peer reviewed as Gospel. If a pre-requesite to publishing is membership, money, qualifications, contacts, support, etc then there is a possibility that the data pool is biased. If there is some sort of selection process involved before publishing, then there is a possibility that not all studies have been granted equal treatment. One needs to know the answers to these questions before accepting the validity of the argument that 98% of scientists are in agreement on the cause of climate change.
I've often been confounded by how they come up with a global temperature. There just seems to be so many variables. Are they talking about maximum temps, minimum temps, averages of temps taken every minute, hour, day or week? Are they using the same methods now as they were in the beginning and throughout the lifecycle of the data collectiojn period? Are they taking them equally over land and sea? Are they taken equidistant from eachother both horizontally and vertically?
Obviously there is much more to say on the subject of integrity but I think you get my point. At the end of the day, the best way to prove if climate change is our fault, would be to determine if it's happening on the other planets in the solar system as well. Can we reliably determine this? I don't know.
As far as doing something about climate change. Well, you may say it doesn't matter who is to blame, but that is quite a significant point. If we are to blame, then we can definitely do something about it and should be acting immediately. However, if we are not to blame, then it is futile to think that we can do anything to stop it. Pretty important point, don't you think? The good news is that we can actually do a lot to reduce the burning of fossil fuels without negatively impacting on the population significantly, just in case it is all our fault. There is already ample evidence out there to suggest that fossil fuel burning is having a detrimental impact on our planet in non-climate change ways. So a move away from fossil fuels is to be encouraged, especially when we have this huge source of free energy called the Sun, just sitting there each day. I just don't think imposing a huge tax on carbon usage is a smart move, when so many alternatives exist that can produce the same outcome, such as encouraging innovation and exploration in new technologies that favour renewable energy over fossil fuels.
Now we can pretend, that we don't know what the answers are, but the reality is, none of the answers either make enough money or preserve enough control for those in power to make it worthwhile implementing.
That would be interesting if oceans were getting less acidic and carbon is being removed from the ocean and deposited into the atmosphere, but that is the opposite of observed fact. Of course there is a turnover of man-emitted and pre-human atmospheric carbon to and from the ocean & atmosphere.
I am not saying that there must be less dissolved CO2 in the oceans. The oceans can release CO2 when warmed and at the same time absorb more human CO2 than they have released, as pointed out on page 26. But my argument here is that some of the CO2 increase is probably natural. I think the assumption that human emissions are solely responsible for the entire atmospheric CO2 increase is doubtful, especially in view of 1850 coming roughly 800 years after the start of the MWP, i.e. when we would expect CO2 concentrations to start rising naturally anyway on the empirical basis of the ice-core record. The oceans are assumed to have warmed since 1850 and that warming should have released some CO2 into the atmosphere too (see calculations by Jaworowski on previous page).
Just one publication. I don't know the details of what really went into this but the idea that oceans are acidifying as a commonly understood observational fact is not based on one observation from Hawaii. Really, do you believe professional scientists are that naive?
I was after time-series. That NOAA map is from 2005 and does not show PCO2(aq) concentrations changing over years. In any case, the paper you referenced did mention some time-series:
Detailed tests may be made by comparing the monthly observations made during the Bermuda Atlantic Time Series (BATS) program (Bates et al., 2012), the Hawaii Ocean Time Series (HOT) program (Dore et al., 2009; Fujieki et al., 2012), and the European Time Series in the Canary Islands (ESTOC) program (Santana-Casiano et al., 2007; Gonzalez-Davila and Santana-Casiano, 2009).
edit on 24-7-2016 by Nathan-D because: (no reason given)
originally posted by: Nathan-D
That would be interesting if oceans were getting less acidic and carbon is being removed from the ocean and deposited into the atmosphere, but that is the opposite of observed fact. Of course there is a turnover of man-emitted and pre-human atmospheric carbon to and from the ocean & atmosphere.
I am not saying that there must be less dissolved CO2 in the oceans. The oceans can release CO2 when warmed and at the same time absorb more human CO2 than they have released, as pointed out on page 26. But my argument here is that some of the CO2 increase is probably natural.
If oceans release additional carbon from global warming from human activity [they are absorbing at present], then that is a positive feedback to increase sensitivity of anthropogenic greenhouse emissions.
I wouldn't call that 'natural'.
The physics of the ocean in this respect (higher temperatures lower ability of ocean to absorb additional carbon) mean that the problem will accelerate now and in the future. It is is bad news.
I think the assumption that human emissions are solely responsible for the entire atmospheric CO2 increase is doubtful,
Historical human emissions have been substantially larger than the atmospheric CO2 increase.
Where the molecule of carbon eventually came from originally is not primary to the effect of climate change through radiative transfer---if human global warming (of which CO2 is just one albeit majority contributor) makes the oceans hotter and the equilibrium carbon absorption goes down (as it does) then that's just being extra fuxxored.
edit on 24-7-2016 by mbkennel because: (no reason given)
If oceans release additional carbon from global warming from human activity [they are absorbing at present], then that is a positive feedback to increase sensitivity of anthropogenic greenhouse emissions.
Well then I suppose this leads us back to the fundamental question: how much radiation-enhancement is CO2 actually producing and is it the main cause of global warming? I personally disagree with the claim that CO2 is the main cause of global warming. That is a legitimate position to take in this open, public debate, is it not? (I accept that human CO2 causes warming and that global warming is happening, which is more than some, and so I wouldn’t really call myself a denier).
edit on 24-7-2016 by Nathan-D because: (no reason given)
new topics
-
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 2 hours ago -
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 8 hours ago -
Maestro Benedetto
Literature: 10 hours ago -
Is AI Better Than the Hollywood Elite?
Movies: 10 hours ago
top topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies: 8 hours ago, 28 flags -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues: 14 hours ago, 8 flags -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest: 16 hours ago, 7 flags -
Las Vegas UFO Spotting Teen Traumatized by Demon Creature in Backyard
Aliens and UFOs: 13 hours ago, 6 flags -
A Warning to America: 25 Ways the US is Being Destroyed
New World Order: 2 hours ago, 6 flags -
Meadows, Giuliani Among 11 Indicted in Arizona in Latest 2020 Election Subversion Case
Mainstream News: 16 hours ago, 5 flags -
2024 Pigeon Forge Rod Run - On the Strip (Video made for you)
Automotive Discussion: 14 hours ago, 4 flags -
Is AI Better Than the Hollywood Elite?
Movies: 10 hours ago, 3 flags -
The functionality of boldening and italics is clunky and no post char limit warning?
ATS Freshman's Forum: 16 hours ago, 1 flags -
Maestro Benedetto
Literature: 10 hours ago, 1 flags
active topics
-
President BIDEN's FBI Raided Donald Trump's Florida Home for OBAMA-NORTH KOREA Documents.
Political Conspiracies • 19 • : ADVISOR -
When an Angel gets his or her wings
Religion, Faith, And Theology • 22 • : AcrobaticDreams1 -
King Charles 111 Diagnosed with Cancer
Mainstream News • 321 • : FlyersFan -
Is there a hole at the North Pole?
ATS Skunk Works • 41 • : burritocat -
Massachusetts Drag Queen Leads Young Kids in Free Palestine Chant
Social Issues and Civil Unrest • 16 • : FlyersFan -
Gaza Terrorists Attack US Humanitarian Pier During Construction
Middle East Issues • 33 • : FlyersFan -
Weinstein's conviction overturned
Mainstream News • 24 • : burritocat -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 690 • : burritocat -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 266 • : SchrodingersRat -
New whistleblower Jason Sands speaks on Twitter Spaces last night.
Aliens and UFOs • 66 • : baablacksheep1
