It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
3
share:
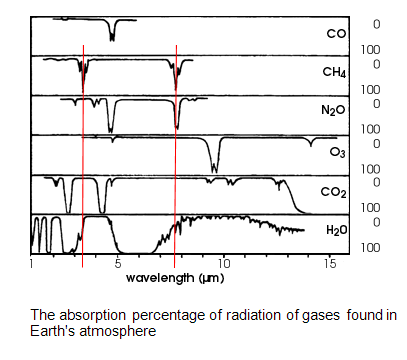
CH4’s absorption bands overlap with those of water vapor. CH4 is thus irrelevant. In other words, any energy that the methane might have absorbed has already been absorbed by H2O.
There is really nothing to worry about, scientifically. The main thing to worry about is over-reacting politicians and brainwashed citizens who vote for them.
edit on 13-9-2018 by OneArmedBandit because: (no reason given)
a reply to: OneArmedBandit
Water vapor and free oxygen molecules negate the atmospheric effect of CH4 molecules, so, yes, you are correct that, for the most part, CH4 is a rather irrelevant catalyst in the overall long-term atmospheric drivers of temperature changes on earth.
ETA: In large enough quantities, though, it can have a temporary effect on localized weather.
Water vapor and free oxygen molecules negate the atmospheric effect of CH4 molecules, so, yes, you are correct that, for the most part, CH4 is a rather irrelevant catalyst in the overall long-term atmospheric drivers of temperature changes on earth.
ETA: In large enough quantities, though, it can have a temporary effect on localized weather.
edit on 13-9-2018 by SlapMonkey because: (no
reason given)
originally posted by: OneArmedBandit
CH4’s absorption bands overlap with those of water vapor. CH4 is thus irrelevant. In other words, any energy that the methane might have absorbed has already been absorbed by H2O.
You are attempting to draw vast conclusions from a half-vast understanding of the information on that chart.
The fact that water vapor shows near 100% absorption at the same location on the spectrum that Methane also shows a near 100% absorption does NOT mean that water vapor absorbs 100% of the IR energy passing through a volume of air. Those absorption bands are on a per molecule basis. The way to interpret that chart is to realize that a quantum of IR radiation that has a wavelength right at one of the peaks for Methane would have a near 100% probability of being absorbed by the Methane molecule if that quantum of light happened to hit the Methane molecule. Similarly, if that same quantum of IR light happened to hit a water molecule in the air it would also have a near 100% probability of being absorbed by that water molecule. Obviously, any body of air could have a mixture of Methane and water vapor in it and any IR of the correct wavelength passing through it would be absorbed by whichever of those molecules it happened to hit.
If your interpretation were correct, then that chart would be saying that Methane absorbs 100% of the IR energy in the atmosphere at that wavelength AND water vapor also absorbs 100% of IR energy in the atmosphere at that wavelength. That would clearly be impossible, since no mixture of Methane and water vapor could absorb twice as much energy as is actually in the atmosphere.
The sum total of all greenhouse gases in the Earth's atmosphere does not absorb 100% of the IR energy passing through. If it did, Earth would resemble Venus. Some fraction of the IR coming off the Earth's surface escapes to space without being absorbed. If that were not true, then adding greenhouse gases to the air would not increase the temperature because all of the IR passing through would already have been absorbed.
It is true that water vapor is a strong greenhouse gas. It is estimated to account for around 60% of the total heat trapped by greenhouse gases on Earth. The number varies from time to time and from location to location, because the column density of water vapor in the atmosphere is not constant. That's because water vapor is a condensible gas. If the air temperature goes down at a particular location and time, then that body of air will precipitate out the water content. If the air temperature goes up at a particular location and time, then that body of air can contain more water vapor.
Carbon Dioxide and Methane do not precipitate out at normal Earth atmospheric pressures and temperatures, When they accumulate in the atmosphere they create a detectable rise in the atmospheric temperature just because they are directly absorbing IR energy. However, that temperature rise has the added effect of increasing the solubility of water vapor in the air, which is also a greenhouse gas. In other words, the increased concentration of water vapor in the air due to an increase in the background concentrations of Carbon Dioxide and Methane creates positive feedback and amplifies the greenhouse effect of those two non-precipitable gases.
China and Africa are planting vast bands of trees to try and reverse desertification. Lol, those are the bands I thought you were talking about... I
guess not.
a reply to: 1947boomer
More like in your words.
Could well work the other way, increased cloud cover increasing albedo.
In other words, the increased concentration of water vapor in the air due to an increase in the background concentrations of Carbon Dioxide and Methane creates positive feedback and amplifies the greenhouse effect of those two non-precipitable gases.
More like in your words.
Could well work the other way, increased cloud cover increasing albedo.
originally posted by: OneArmedBandit
a reply to: SlapMonkey
Free oxygen molecules?
Are you trying to say O2 is a greenhouse gas?
No, what I said was:
Water vapor and free oxygen molecules negate the atmospheric effect of CH4 molecules, so, yes, you are correct that, for the most part, CH4 is a rather irrelevant catalyst in the overall long-term atmospheric drivers of temperature changes on earth.
I'm not sure where that leaves you room to question if I was calling oxygen a greenhouse gas, but I didn't say that at all, or even imply it. In fact, the point was that oxygen reduces the impact of CH4 in the greenhouse cycle because of its chemical reaction negates the lifespan and therefore the warming duration of CH4 in the atmosphere.
More directly: No, O2 is not a greenhouse gas.
The equation below was apparently inferred with an Atmospheric Emitted Radiance Interferometer (AERI) to determine how much warming methane (CH4)
causes. An Atmospheric Emitted Radiance Interferometer (AERI) is a ground-based instrument that measures the down-welling infrared radiance from the
Earth’s atmosphere.
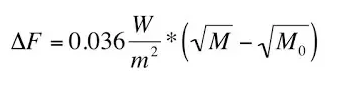
Where ΔF stands for ‘Radiative Forcing’; M0 is the initial CH4 concentration; M is the final CH4 concentration, and W/m2 stands for ‘watts per square metre’. CH4 is assumed to have increased from its pre-industrial level of 700ppb (parts per billion) to 1800ppb. Therefore the equation above gives us a radiative forcing of 0.57 W/sq.m. By comparison, CO2 is assumed to have caused a radiative forcing of around 2 W/sq.m since 1850. So, while it’s not causing anywhere near as much as CO2, it’s not completely inconsequential either.
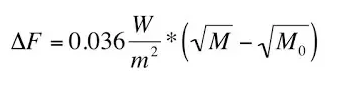
Where ΔF stands for ‘Radiative Forcing’; M0 is the initial CH4 concentration; M is the final CH4 concentration, and W/m2 stands for ‘watts per square metre’. CH4 is assumed to have increased from its pre-industrial level of 700ppb (parts per billion) to 1800ppb. Therefore the equation above gives us a radiative forcing of 0.57 W/sq.m. By comparison, CO2 is assumed to have caused a radiative forcing of around 2 W/sq.m since 1850. So, while it’s not causing anywhere near as much as CO2, it’s not completely inconsequential either.
new topics
-
They say justice is blind.
Religion, Faith, And Theology: 19 minutes ago -
Liberal Democrats to Table a Motion of No Confidence in the government Tomorrow
Regional Politics: 2 hours ago -
Time Traveler Caught on Camera?
Paranormal Studies: 2 hours ago -
Trump legal cases are falling apart at break neck speeds
US Political Madness: 2 hours ago -
We are screwed
Global Meltdown: 2 hours ago -
Medvedev: “No one can hide, a global catastrophe is coming”
World War Three: 3 hours ago -
Russians And Americans At Same Airbase In Niger But Not Sharing Restrooms
World War Three: 6 hours ago -
US Air Force Secretary Kendall flies in cockpit of plane controlled by AI
Aircraft Projects: 11 hours ago
top topics
-
Trump legal cases are falling apart at break neck speeds
US Political Madness: 2 hours ago, 11 flags -
Medvedev: “No one can hide, a global catastrophe is coming”
World War Three: 3 hours ago, 10 flags -
We are screwed
Global Meltdown: 2 hours ago, 9 flags -
The BEAST System of Revelation has been awoken and has assumed control, at least since COVID.
New World Order: 16 hours ago, 7 flags -
Time Traveler Caught on Camera?
Paranormal Studies: 2 hours ago, 4 flags -
Messages of Hope – Unity through AI
Dreams & Predictions: 12 hours ago, 3 flags -
Liberal Democrats to Table a Motion of No Confidence in the government Tomorrow
Regional Politics: 2 hours ago, 3 flags -
Russians And Americans At Same Airbase In Niger But Not Sharing Restrooms
World War Three: 6 hours ago, 2 flags -
Happy Cinco de mayo.
General Chit Chat: 12 hours ago, 2 flags -
US Air Force Secretary Kendall flies in cockpit of plane controlled by AI
Aircraft Projects: 11 hours ago, 1 flags
active topics
-
Candidate TRUMP Now Has Crazy Judge JUAN MERCHAN After Him - The Stormy Daniels Hush-Money Case.
Political Conspiracies • 1214 • : WeMustCare -
Medvedev: “No one can hide, a global catastrophe is coming”
World War Three • 39 • : ufoorbhunter -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 817 • : cherokeetroy -
SC Jack Smith is Using Subterfuge Tricks with Donald Trumps Upcoming Documents Trial.
Dissecting Disinformation • 131 • : WeMustCare -
Time Traveler Caught on Camera?
Paranormal Studies • 8 • : CarlLaFong -
Modern Mind Control
General Conspiracies • 41 • : Dreftenq -
The BEAST System of Revelation has been awoken and has assumed control, at least since COVID.
New World Order • 13 • : Cvastar -
They say justice is blind.
Religion, Faith, And Theology • 0 • : BrotherKinsMan -
We are screwed
Global Meltdown • 5 • : Cvastar -
Mysterious Spiral 'UFO' Sightings Reported Across US, Europe
Aliens and UFOs • 21 • : pianopraze
3
