It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: Grimpachi
a reply to: Vector99
Don't bother. I feel kind of bad for you now because you really don't know these things so here is a bone.
Read this it will explain list all he differing reasons for varying sea level rise.
The Secret of Sea Level Rise: It Will Vary Greatly by Region
I especially like the "polar ice caps" will pull all the water near them closer....
originally posted by: raymundoko
8.3 to 8.2 is not acidification. To think it is shows a complete lack in understanding of chemistry. The proper term is neutralizing. The term acidification in reference to the ocean was coined out of propaganda to incite fear. The ocean would only be acidifying if the PH level was at 7 and trending down.
This is an example of debating semantics: the oceans are getting less alkaline. In this case 'acidification' describes the increase in the level of acidity in the oceans. The 200+ scientists that signed the document I linked have no problem calling it so because it is what's happening: when CO2 is added to seawater it reacts with the water to form carbonic acid. This means that acid is being added hence the term acidification.
You can show me a PH scale all you want, I work with them, but what's happening in the oceans is not as simple as dipping a urine stick in a sample, it's more complicated because sea life is very sensitive to even the smallest change.
And LOL at the ancient climate! At least I'm not the one who thinks a 'tropical Earth' will be great for us!!
originally posted by: ParasuvO
originally posted by: Grimpachi
a reply to: Agartha
Don't you love it when people try to use climate from over a 145 million years ago and when the continents were still forming to compare to today's warming?
I love hearing people tell us that they know when and where extinction level events occurred, and how many millions and billions of years it all takes..........
WITH NEVER ONE SHRED OF PROOF.
And they believe it just as much if not MORE than the most bought in religious follower ever.
They call them fossils and people that dig them up are called palentologists. My son went through this in 3rd grade. See with fossils there once living creatures that go through a process called carbonization. I tried to keep it simple for you since you didn't understand how they can tell when extinct ions occured. Hope that helps.
originally posted by: Agartha
This is an example of debating semantics: the oceans are getting less alkaline. In this case 'acidification' describes the increase in the level of acidity in the oceans.
In reality to describe this as "acidification" is not only wrong, but very dishonest.
The movement in ph from 8.4 to 8.3 does not represent "acidification" as there is no increase in the acidity, but rather a reduction in the alkalinity...a subtle, but, important distinction. Only after the ph becomes less than 7.0 can this be described as acid.
To attempt to represent this as an increase of acidity is dishonest to the point of fraud!
originally posted by: ParasuvO
originally posted by: Grimpachi
a reply to: Vector99
Don't bother. I feel kind of bad for you now because you really don't know these things so here is a bone.
Read this it will explain list all he differing reasons for varying sea level rise.
The Secret of Sea Level Rise: It Will Vary Greatly by Region
I especially like the "polar ice caps" will pull all the water near them closer....
Yeah, I liked that too...
Did you know that a glacier has less gravity (and mass) the an equal volume of liquid water?
Its true, so I guess those glaciers, and ice sheets have less "pull" than suspected!
edit on 5-12-2015 by tanka418 because: (no reason given)
a reply to: tanka418
What is clear is you do not understand basic chemistry and have resorted to arguing semantics.
The PH scale goes from 0 to 14, now lets look at that like a number line with 0 being on the left and 14 on the right.
When the PH figure shifts to the left on said number line, a solution is said to become more acidic or less basic(those terms are interchangeable).
8.3 is to the left of 8.4 on said number line....
What is clear is you do not understand basic chemistry and have resorted to arguing semantics.
The PH scale goes from 0 to 14, now lets look at that like a number line with 0 being on the left and 14 on the right.
When the PH figure shifts to the left on said number line, a solution is said to become more acidic or less basic(those terms are interchangeable).
8.3 is to the left of 8.4 on said number line....
edit on 5-12-2015 by jrod because: typo
originally posted by: jrod
a reply to: tanka418
What is clear is you do not understand basic chemistry and have resorted to arguing semantics.
The PH scale goes from 0 to 14, now lets look at that like a number line with 0 being on the left and 14 on the right.
When the PH figure shifts to the left on said number line, a solution is said to become more acidic or less basic(those terms are interchangeable).
8.3 is to the left of 8.4 on said number line....
So...you prefer the fraudulent approach then...
Here a wee definition to help illustrate:
Acid
1.containing acid or having the properties of an acid; in particular, having a pH of less than 7
At a pH of 8.x a substance is alkali (basic) and cannot be described as "acidic".
Alkali (base)
1.having the properties of an alkali, or containing alkali; having a pH greater than 7.
edit on 5-12-2015 by tanka418 because: (no reason given)
a reply to: boymonkey74
Probably a good idea. We don't need kids born into families with weird notions. We have enough weird people now.
Probably a good idea. We don't need kids born into families with weird notions. We have enough weird people now.
originally posted by: tanka418
In reality to describe this as "acidification" is not only wrong, but very dishonest.
The movement in ph from 8.4 to 8.3 does not represent "acidification" as there is no increase in the acidity, but rather a reduction in the alkalinity...a subtle, but, important distinction. Only after the ph becomes less than 7.0 can this be described as acid.
To attempt to represent this as an increase of acidity is dishonest to the point of fraud!
~sigh~
Re-read my post and the link I provided although Jrod has explained it clearly too. I won't repeat my words, they were very simple to understand... but let me try to help you by adding this:
The PH scale is a logarythm which means that PH7 is ten times more acidic than PH8 and when we talk about living organisms even a change of 0.1 can kill them. Look at us: our blood PH has to be between 7.45 and 7.35, the smallest change can cause seizures and deaths. The ocean is not acidic as on being on PH7: the ocean is becoming MORE acidic hence experts use the term 'acidification'. CO2 is adding acid to the oceans, hence they use that term. If you don't understand I don't think I can explain it in any other way. Or go and sue them for fraud, the link is below.
Here: ocean-acidification.net...
Read the Monaco document that was signed by 320 experts from all over the world
edit on 6-12-2015 by Agartha because: Added link.
I had done figures using the old farmers almanac and temperature differences and yeah knew it prior just like to have backing. The world bank group is
the ring leader IMF seem to be in the mix as well but the world banking group is UN backed and definite leader in this also what there goal is sub
stainable dependency.
Basically loaning lots and lots of money to countries when they can't pay the bill they grab land resources ect. They have been to court more anyone over this too. So yeah don't fall for any of this they have already imposed carbon tax on the UE and others and the push is for us. I think from the way it's written out that it's already on us just hasn't been implemented sounds like it has all been signed away.
Basically loaning lots and lots of money to countries when they can't pay the bill they grab land resources ect. They have been to court more anyone over this too. So yeah don't fall for any of this they have already imposed carbon tax on the UE and others and the push is for us. I think from the way it's written out that it's already on us just hasn't been implemented sounds like it has all been signed away.
originally posted by: Agartha
~sigh~
Re-read my post and the link I provided although Jrod has explained it clearly too. I won't repeat my words, they were very simple to understand... but let me try to help you by adding this:
sigh! Indeed!
I don't need to reread your post, I know what it says, and why...
The PH scale is a logarythm which means that PH7 is ten times more acidic than PH8 and when we talk about living organisms even a change of 0.1 can kill them. Look at us: our blood PH has to be between 7.45 and 7.35, the smallest change can cause seizures and deaths. The ocean is not acidic as on being on PH7: the ocean is becoming MORE acidic hence experts use the term 'acidification'. CO2 is adding acid to the oceans, hence they use that term. If you don't understand I don't think I can explain it in any other way. Or go and sue them for fraud, the link is below.
Read the Monaco document that was signed by 320 experts from all over the world
Well, ignoring the irrelevant in your post; Technically speaking a pH of 7.0 is not more "acidic" than a pH of 7.x (x> 0). This is true because it is considered neutral (the pH of intrinsic water = 7.0), and thus is neither more acidic, nor basic in nature.
The representation given by your demi-god scientists is a deliberate and inappropriate "warping" of the meaning of several concepts and accepted scientific thought. If either you or jrod had more than high school chemistry you would have recognized the deception rather quickly.
I only took a couple of years of college Chemistry, and dinosaurs roamed when I did, but, as I remember; water, or indeed any other substance absorbs gasses at rather fixed rates based on pressure, as opposed to ambient concentrations...just sayin'.
No...I don't need to read your Monaco document signed by 320 liars.
You need to understand that, unlike you, I don't rely on some unknown name for my knowledge; I rely on my education (formal and informal) and experience...
originally posted by: Agartha
This is an example of debating semantics: the oceans are getting less alkaline. In this case 'acidification' describes the increase in the level of acidity in the oceans. The 200+ scientists that signed the document I linked have no problem calling it so because it is what's happening: when CO2 is added to seawater it reacts with the water to form carbonic acid. This means that acid is being added hence the term acidification.
Some thing I didn't see your scientists mention; the absorption rate hasn't changed over time. Which is to say; that as long as the atmospheric pressure has been what it currently is, the amount of CO2 absorbed by the Oceans (and all other exposed water)has been constant (hasn't changed). Thus, no additional carbonic acid is produced. And, that factor of oceanic pH remains in original equilibrium. Thus it is a null point.
You also failed to indicate that only a rather small amount of carbonic acid is ever produced by this process.
So this is far from semantic distortion...it is a deliberate falsification of the realities of Oceanic pH.
originally posted by: tanka418
If either you or jrod had more than high school chemistry you would have recognized the deception rather quickly.
I only took a couple of years of college Chemistry...
Every post you make has to have a paragraph on your university education and how higher it is than everybody else's. You did the same on another thread, every time I told you Pye and Forester were charlatans you told me I should have gone to college to understand their 'science'. I told you already: I am a 45 year old woman with a degree in Medical Sciences, with lots of chemistry actually. I say it again: we are not discussing our curriculm vitae here. Stay on topic.
originally posted by: tanka418
Some thing I didn't see your scientists mention; the absorption rate hasn't changed over time. Which is to say; that as long as the atmospheric pressure has been what it currently is, the amount of CO2 absorbed by the Oceans (and all other exposed water)has been constant (hasn't changed). Thus, no additional carbonic acid is produced. And, that factor of oceanic pH remains in original equilibrium. Thus it is a null point.
You also failed to indicate that only a rather small amount of carbonic acid is ever produced by this process.
So this is far from semantic distortion...it is a deliberate falsification of the realities of Oceanic pH.
Wrong. The absorption depends on the excess carbon. Oceans absorb aprox 25% of all CO2, the higher the level of CO2, the higher the level the oceans absorb. This is why ocean's PH has been decreasing.
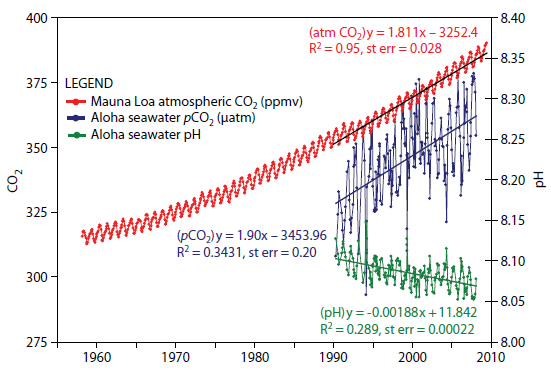
From: journals.ametsoc.org...
No...I don't need to read your Monaco document signed by 320 liars.
Liars? You believe in the 'science' of Pye and Forester and call hundred of experts liars?
originally posted by: Agartha
Wrong. The absorption depends on the excess carbon. Oceans absorb aprox 25% of all CO2, the higher the level of CO2, the higher the level the oceans absorb. This is why ocean's PH has been decreasing.
I take it you've not heard of Henry's Law then...which along with a little more chemistry, some physics, and a bit of math give us:
x = Py÷H
where
x= mol fraction in water
Py = partial pressure in gas (e.g., total pressure × mol fraction), atm
H = Henry's law solubility constant. At 30C Henry's constant for CO2=1900
Henry's Constant is entirely dependent on temperature and pressure...
As far as I know the atmospheric pressure is remaining relatively constant; thus the absorption rate of CO2 into water remains unchanged, demonstrating that your scientists are likely attempting to use linguistics, and communications to "pull the wool over your eyes". (Isn't that what a lie is?)
Liars? You believe in the 'science' of Pye and Forester and call hundred of experts liars?
Ya know...after that little bit of hypocrisy of yours, I'm kind of surprised you mentioned that.
And, no actually, I don't "believe" in "Pye's science"...I believe in the science I learned in school, and on the job...you know the "stuff" successful careers are made of...
BTW; there is, in reality, only one science!
Question for you; this science you are so "on" about; Why is it that chemists and physicists denounce it as a fraud, and only climatologists, and the uninitiated are the proponents?
And, yes, I'd call your scientists liars; simply because they are attempting to peddle snake oil!
This whole "global warming" / "climate change" due to Humans, is only yet another way for the rich and powerful to remain that way. In reality; while there is a change occurring in the climate, it is not due to unnatural elements, nor, is it likely to ever become a serious issue.
So...it all boils down to honesty; the climate science types are being dishonest and attempting to establish a fabrication as fact and science...fortunately real science doesn't work that way. Unfortunately there are people like you who only look at the politics, and refuse to test the science.
a reply to: tanka418
Organic chem was my highest level of chemistry... more than chem 101.
The 40% rise of CO2 is something that cant be ignored.
I see that you are calling scientists liars now.
Do you really believe that the scientists at NASA and NOAA are lying to us to 'keep the rich and powerful, rich and in power'?
It appears your arguments are getting more delusional with each post.
Organic chem was my highest level of chemistry... more than chem 101.
The 40% rise of CO2 is something that cant be ignored.
I see that you are calling scientists liars now.
Do you really believe that the scientists at NASA and NOAA are lying to us to 'keep the rich and powerful, rich and in power'?
It appears your arguments are getting more delusional with each post.
originally posted by: jrod
a reply to: tanka418
Organic chem was my highest level of chemistry... more than chem 101.
Hmmmmm...If I remember correctly year 2 was Chem 2xx...
I see that you are calling scientists liars now.
Do you really believe that the scientists at NASA and NOAA are lying to us to 'keep the rich and powerful, rich and in power'?
In some specific instances, yes, I'm calling scientists liars.
The scientists at NASA, along with their engineers are idots! As evidence I would preset their greatest recent achievements. You know things like "New Horizons" and other now famous spacecraft. When I think of the actual, real world technology available at the time of deployment of those robots, and compare it to what was actually deployed...I get a little sick to my stomach.
They have done such a wonderful job of collecting and returning dat, but, they could have returned vastly more and vastly more detailed data by simply using the available technology. The worst of it is; they will tell you straight faced that what they sent was the very best technology at the time...btw, that would be a lie.
I'm sure that IF you talked to a NASA astronomer I'm sure he will tell you he works with and has the very best of data and datasets...that would e a lie...especially when it comes to "how" NASA, and many other scientists organize their databases...seriously...using text to represent numerical values, base 60 values to represent decimals...
Y'all are trying to defend something you have no real knowledge of...
As for NOAA; I used to have respect for them, but that has become kind of tarnished as of late...
originally posted by: tanka418
Henry's Constant is entirely dependent on temperature and pressure...
As far as I know the atmospheric pressure is remaining relatively constant; thus the absorption rate of CO2 into water remains unchanged...
Wrong!
Henry's constant: "the concentration of a dissolved gas in a solution is directly proportional to the partial pressure of that gas above the solution".
The partial pressure of carbon dioxide is the gas phase pressure (i.e. in the air above a waterway) of carbon dioxide which would be in equilibrium with the dissolved carbon dioxide =
an increase in the concentration of CO2 in the atmosphere directly leads to an increase in the amounts of CO2 absorbed by the oceans.
Partial pressure is not atmospheric pressure but that particular gas pressure above that particular water.
the climate science types are being dishonest and attempting to establish a fabrication as fact and science...fortunately real science doesn't work that way. Unfortunately there are people like you who only look at the politics, and refuse to test the science.
LOL ... That's all I do: study, analyze and test the science. My only wish is for people to start using green energy, to stop wasting resources, to stop polluting the planet. That's all I want: a green future for my children.
originally posted by: Agartha
Wrong!
lol
Henry's constant: "the concentration of a dissolved gas in a solution is directly proportional to the partial pressure of that gas above the solution".
The partial pressure of carbon dioxide is the gas phase pressure (i.e. in the air above a waterway) of carbon dioxide which would be in equilibrium with the dissolved carbon dioxide =
an increase in the concentration of CO2 in the atmosphere directly leads to an increase in the amounts of CO2 absorbed by the oceans.
Partial pressure is not atmospheric pressure but that particular gas pressure above that particular water.
Kind of interesting how you start off like you have an understanding. Then your logic breaks, and you go off on some tangent that ends in a complete breakdown of understanding...
So you think about this: How does the concentration of CO2 in water increase when there is no corresponding increase in partial pressure.
new topics
-
Jan 6th truth is starting to leak out.
US Political Madness: 18 minutes ago -
Deep state control - How your tax dollars are used to censor and brainwash
Propaganda Mill: 1 hours ago -
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News: 1 hours ago -
Top Sci Fi/Horror Crossover Movies
Movies: 4 hours ago -
Magic Vaporizing Ray Gun Claim - More Proof You Can't Believe Anything Hamas Says
War On Terrorism: 4 hours ago -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies: 5 hours ago -
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness: 5 hours ago
top topics
-
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness: 5 hours ago, 14 flags -
Magic Vaporizing Ray Gun Claim - More Proof You Can't Believe Anything Hamas Says
War On Terrorism: 4 hours ago, 6 flags -
Top Sci Fi/Horror Crossover Movies
Movies: 4 hours ago, 6 flags -
What Am I Hearing
General Chit Chat: 14 hours ago, 4 flags -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies: 5 hours ago, 4 flags -
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News: 1 hours ago, 4 flags -
Jan 6th truth is starting to leak out.
US Political Madness: 18 minutes ago, 4 flags -
Deep state control - How your tax dollars are used to censor and brainwash
Propaganda Mill: 1 hours ago, 0 flags
active topics
-
DONALD J. TRUMP - TIME's Most Extraordinary Person of the Year 2024.
Mainstream News • 7 • : Ravenwatcher -
Jan 6th truth is starting to leak out.
US Political Madness • 2 • : DAVID64 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 3643 • : Thoughtful3 -
President-Elect DONALD TRUMP's 2nd-Term Administration Takes Shape.
Political Ideology • 306 • : Oldcarpy2 -
USS Liberty - I had no idea. Candace Owen Interview
US Political Madness • 15 • : network dude -
Top Sci Fi/Horror Crossover Movies
Movies • 5 • : Roma1927 -
One out of every 20 Canadians Dies by Euthanasia
Medical Issues & Conspiracies • 13 • : BeyondKnowledge3 -
A Bunch of Maybe Drones Just Flew Across Hillsborough County
Aircraft Projects • 29 • : Macenroe82 -
Thank you to people who said they were going to do something to people they found voted for Trump.
US Political Madness • 26 • : Blackstone0523 -
FBI Director CHRISTOPHER WRAY Will Resign Before President Trump Takes Office on 1.20.2025.
US Political Madness • 20 • : WeMustCare
