It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
University of Bristol are working on something similar. Idea has been mooted batteries using nuclear waste could revolutionise use of photovoltaics,
meaning truly off-grid energy would be possible, even for urban dwellers and businesses.
Scientists have discovered a way to convert nuclear waste into radioactive black diamond batteries which last more than 5,000 years.
Researchers at the University of Bristol have found a means of creating a battery capable of generating clean electricity for five millennia, or as long as human civilization has existed.
Scientists found that by heating graphite blocks – used to house uranium rods in nuclear reactors – much of the radioactive carbon is given off as a gas.
This can then be gathered and turned into radioactive diamonds using a high-temperature chemical reaction, in which carbon atoms are left on the surface in small, dark-colored diamond crystals.
These man-made diamonds produce a small electrical charge when placed near a radioactive source.
The radioactive diamonds are then encased safely within a layer of non-radioactive diamond. The surface of a complete diamond emits less radiation than a banana.
eclinik.net...-8877
It is not a battery.
It is a form of fuel cell.
How would you charge it?
It is a form of fuel cell.
How would you charge it?
a reply to: RussianTroll
Better hope its stable, else does it not blow up in a helicopter in the end Knight and Day style?
Better hope its stable, else does it not blow up in a helicopter in the end Knight and Day style?
edit on 23-8-2020 by andy06shake because:
(no reason given)
It's not your typical battery, but that's what we call it, specifically a type of atomic battery called a betavoltaic battery.
originally posted by: charlyv
It is not a battery.
It is a form of fuel cell.
How would you charge it?
Atomic battery
An atomic battery, nuclear battery, tritium battery or radioisotope generator is a device which uses energy from the decay of a radioactive isotope to generate electricity. Like nuclear reactors, they generate electricity from nuclear energy, but differ in that they do not use a chain reaction.
That article lists a lot of different types of atomic batteries. The specific type is a non-thermal, betavoltaic battery:
That even mentions the Russian design based on nickel-63 specifically.
Betavoltaic devices, also known as betavoltaic cells, are generators of electric current, in effect a form of battery, which use energy from a radioactive source emitting beta particles (electrons)...
In 2018 a Russian design based on 2-micron thick nickel-63 slabs sandwiched between 10 micron diamond layers was introduced.
It's not a fuel cell according to this definition of a fuel cell:
Fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen) and an oxidizing agent (often oxygen[1]) into electricity through a pair of redox reactions.
The atomic battery doesn't combine fuel with an oxidizing agent to release energy via chemical reaction. It doesn't have anything at all in common with a fuel cell that I can see. The fuel cell is based on chemical energy of the fuel, while the atomic battery is based on atomic energy, not chemical energy.
edit on 2020823 by Arbitrageur because: clarification
a reply to: Arbitrageur
I understand the difference, as there is no oxidizing going on, however it is more of a fuel cell (in its generic form), than it is a battery in that a battery implies a device that can be charged with more electrical current, and a fuel cell creates electrical current by (in this case) converting heat to electricity. The heat in this case is a radioactive process.
I understand the difference, as there is no oxidizing going on, however it is more of a fuel cell (in its generic form), than it is a battery in that a battery implies a device that can be charged with more electrical current, and a fuel cell creates electrical current by (in this case) converting heat to electricity. The heat in this case is a radioactive process.
edit on 24-8-2020 by charlyv because: (no reason given)
You sound confused about batteries, about fuel cells, and about how the Russian design works.
originally posted by: charlyv
a reply to: Arbitrageur
I understand the difference, as there is no oxidizing going on, however it is more of a fuel cell (in its generic form), than it is a battery in that a battery implies a device that can be charged with more electrical current, and a fuel cell creates electrical current by (in this case) converting heat to electricity. The heat in this case is a radioactive process.
Some batteries are rechargeable, some are not. The non-rechargeable batteries have warnings on them to not charge them or they can explode:
13 Investigates exploding alkaline batteries
most alkaline batteries have warnings – albeit tiny ones – telling consumers they could explode. Those warnings instruct not to insert an alkaline battery the wrong direction, expose it to high heat, or charge a non-rechargeable battery. All of those mistakes can prompt explosion.
No, no, I specifically explained in my prior post that the betavoltaic battery is "non-thermal", which should obviously mean it's NOT based on heat as you are incorrectly saying. Some of the other types of atomic batteries are thermal batteries, but not the betavoltaics.
fuel cell creates electrical current by (in this case) converting heat to electricity. The heat in this case is a radioactive process.
if they are designed for micro-electronics then taking into consideration the size of the battery and putting anywhere between 100-1000 batteries and
linking them daisy chaining style, i wonder what kind of outage you would gain from it? enough to act like tony starks miniture arc techology? if its
possible to daisy chain said battery that is, like normal aa's, aaa,s and 9v square batteries...a reply to: RussianTroll
edit on 26-9-2020 by DARREN1976 because: silly wireless keynoard running out of charge and omitting letters!
edit on
26-9-2020 by DARREN1976 because: (no reason given)
Outage is like when your power goes off, so I assume you mean output?
originally posted by: DARREN1976
if they are designed for micro-electronics then taking into consideration the size of the battery and putting anywhere between 100-1000 batteries and linking them daisy chaining style, i wonder what kind of outage you would gain from it? enough to act like tony starks miniture arc techology?
Here's one that's "very powerful" for a betavoltaic ( or claims to be, I think this is a hoax, most are less powerful than this one):

The label says 100 microwatts.
1000 of them would give you 100 milliwatts.
You would need a million of them to just light a single 100 watt light bulb, and I'm sure that Tony Stark needs more than that. That's for the "HOAX" version. For a real battery that's in production, their output is lower, so you'd need more.
The 2.4 V model (2.7V open circuit) is $4500 each (for a real battery):

The power output of the real battery is only 4.2 microwatts:
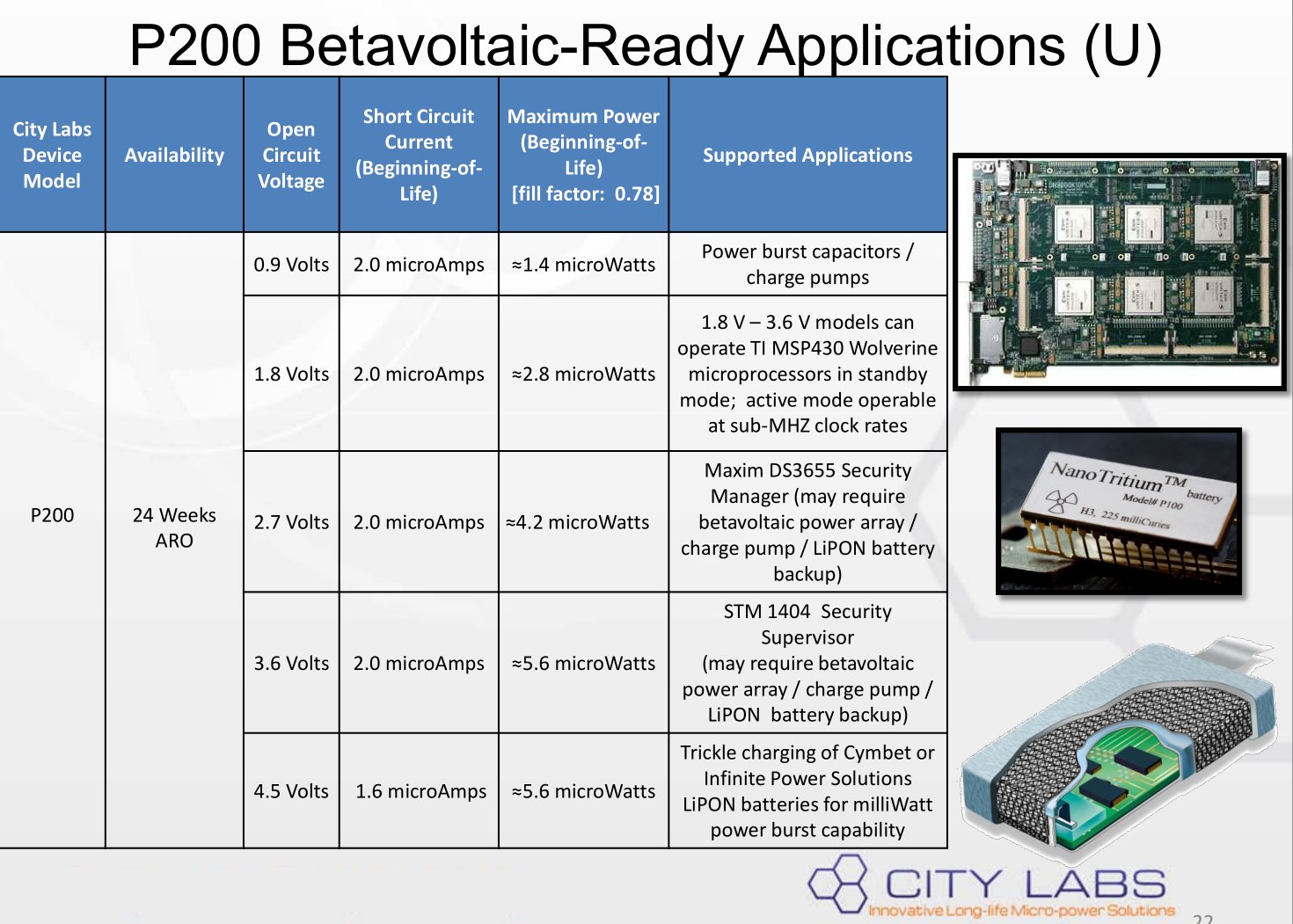
So for the real battery, you'd need about 24 million of those to power a 100 watt light bulb.
24 million x $4500 = $108 Billion
Of course you could run the 100 watt light bulb for 50 years, but still...that's expensive.
Tony Stark's suit needs probably at least 100 times as much power as a 100 watt light bulb, so you're talking at least 10 trillion at current prices, though you can probably get lower cost if you buy in bulk. The other problem is by the time you add up the weight of all those batteries, it would be tons, his suit would never get off the ground.
So this gives some idea of how these will only end up in specialized applications at first. There will probably be improvements in cost, power and efficiency, but they will necessarily be limited, so these will never compete cost-wise with AA alkaline batteries. But if you're launching a satellite, low cost AA is not what you need, you want something to last 50 years without replacement and don't mind spending thousands of dollars since satellites are generally not cheap anyway.
Once it's launched, you can't afford to be going into space to replace the batteries, so you can pay a lot for something that doesn't need replacement. But if the satellite orbits the Earth, a solar panel and rechargeable battery might still be more cost effective. So I think the market is fairly limited, but there probably is a niche market for these batteries.
edit on 2020926 by Arbitrageur because: clarification
originally posted by: RussianTroll
What does Russian "relatively safe for humans " compare to the rest of us? lol JKing
If true, great I'll buy 2.
Haha, I get it. But there are some safety questions though.
originally posted by: Xtrozero
originally posted by: RussianTroll
What does Russian "relatively safe for humans " compare to the rest of us? lol JKing
Presumably, the batteries will be packaged such that the provided shielding makes them "safe" to be around as long as they remain intact. We can be such short term thinkers, some people might not think beyond that.
But if at some point in the future, the shielded package breaks down, it could provide an exposure risk to humans. The nickel powered batteries have the advantage that the radioactive nickel has a half life of only 100 years, so if the package stays intact for 200 years the risk of exposure after that will be small.
The bigger concern is with using carbon/diamond as the power source as discussed in teapot's post, where C14 has a half-life of 5700 years, and the C-14 decomposes into a gas which will build up pressure inside the sealed diamond shell, and could explode or break down the packaging after 200 years, maybe even sooner. Unlike the nickel, which has already been through multiple half-lives after 200 years, the C-14 has barely made a dent in its 5700 year half life after 200 years. If the packaging breaks down and it gets into the food supply, and people eat the C-14, it will kill people. So, safety-wise, we are better off sticking with the nickel for this type of application.
edit on 2020926 by Arbitrageur because: clarification
The main problem with beta decay “batteries” is the amount of voltage produced. You need so many to reach real world applications that you may as
well go whole hog and make a micro reactor like using thorium and some nuclear waste adjunct.
After the power issue is the size. If, say, you need a shoebox sized beta battery to do a 3V job that only runs a few years instead 10,000... well, you use the quarter sized li-ion one.
We had this discussion years ago. Mr Bedlam pointed out the above issues. The “ten times more power” is kind of misleading at these voltages (tenths of milivolts, iirc) is like saying “an increase from 0.000001 to 0.0000011” (or whatever 10% of a million is). That was our sticking point last time.
So thought about real world applications and he said “constantly lighted roads” which leads to more light pollution. Maybe underground tunnels?
It is kind of difficult to think of something that needs to be powered for 10,000 years!!
But at least we are talking about energy storage!
We are such a wasteful society. If we started with trying to conserve all energy that is generated... well, we be in this sh# hole we live in! Really. It is around us and will eventually leach into us humans. That is the true end of the world scenario: drowning in our own filth!
What lovely melancholy!!
After the power issue is the size. If, say, you need a shoebox sized beta battery to do a 3V job that only runs a few years instead 10,000... well, you use the quarter sized li-ion one.
We had this discussion years ago. Mr Bedlam pointed out the above issues. The “ten times more power” is kind of misleading at these voltages (tenths of milivolts, iirc) is like saying “an increase from 0.000001 to 0.0000011” (or whatever 10% of a million is). That was our sticking point last time.
So thought about real world applications and he said “constantly lighted roads” which leads to more light pollution. Maybe underground tunnels?
It is kind of difficult to think of something that needs to be powered for 10,000 years!!
But at least we are talking about energy storage!
We are such a wasteful society. If we started with trying to conserve all energy that is generated... well, we be in this sh# hole we live in! Really. It is around us and will eventually leach into us humans. That is the true end of the world scenario: drowning in our own filth!
What lovely melancholy!!
Maybe so, but it's hard for me to think of these nickel batteries as storage since they are so horribly inefficient. I don't think I've seen a ratio for nickel batteries for how much power you get out as a fraction of the power used to create the battery, however, I'm sure it's a very tiny number. If you find a figure, let me know, but I expect you have to put in more than a million times more power than you get out, maybe one of the worst "storage" ideas ever.
originally posted by: TEOTWAWKIAIFF
But at least we are talking about energy storage!
Nickel-63 is produced by capture on enriched Nickel-62 in High-Flux Isotope Reactor (HFIR) at Oak Ridge National Laboratory. The energy consumption in such a process is huge, and the cost is also high. Thunderf00t said the nickel isotopes costs something like $70,000.00 per gram at time index 10:15 (compare that to about $0.014 per gram for regular, stable nickel). Part of that cost of course reflects the massive amounts of energy used to produce the nickel-63.
Another reason it's hard for me to think of it as storage, is that once you make the Nickel-63 at Oak Ridge or wherever, it's constantly decaying, whether you are using the battery or not. So you could turn the device it powers off, but the "battery" will just keep depleting at the same rate. That's a very undesirable property for a "storage" device, isn't it?
originally posted by: Arbitrageur
But if at some point in the future, the shielded package breaks down, it could provide an exposure risk to humans. The nickel powered batteries have the advantage that the radioactive nickel has a half life of only 100 years, so if the package stays intact for 200 years the risk of exposure after that will be small.
I'm sure they will get the safety part right, you ever open up a lithium battery...I think not...lol
edit on 26-9-2020 by Xtrozero because: (no reason given)
You can't be serious. Those lithium batteries are already releasing toxic chemicals into the environment. People throw them out, they cause fires and the fires release the toxins. They are an example of how we screw things up. Now just imagine adding radioactive materials to those fires, they will release the radioactive toxins into the air, along with all the other toxins the fires are already releasing, but the radioactive toxins are potentially more deadly, especially those with long half-lives that won't go away for thousands of years.
originally posted by: Xtrozero
I'm sure they will get the safety part right, you ever open up a lithium battery...I think not...lol
Exactly what you seem to think makes them safe is part of what makes them unsafe. They are difficult to disassemble so efforts to recycle them properly are impeded because of that, so they end up in trash instead of the recycling stream.
Lithium-ion Batteries are Causing Five-alarm Fires in Garbage Trucks, Waste and Recycling Facilities
In 2017, 65 percent of fires in California waste facilities started with lithium-ion batteries...
In March, a lithium-ion battery sparked a five-alarm fire at a recycling facility in Queens in New York City, which burned for two days. And a recycling plant in Indianapolis shut down after a fire was caused by batteries.
Toxic fluoride gas emissions from lithium-ion battery fires
So, the batteries themselves can release lots of highly toxic substances when they catch fire, but when they do so in the waste stream like garbage trucks, waste processing facilities and landfills, all the other waste with them also burns and additional toxic materials from the other materials in the waste stream are released, which wouldn't be so dangerous without the fire.
The electrolyte in a lithium-ion battery is flammable and generally contains lithium hexafluorophosphate (LiPF6) or other Li-salts containing fluorine. In the event of overheating the electrolyte will evaporate and eventually be vented out from the battery cells. The gases may or may not be ignited immediately. In case the emitted gas is not immediately ignited the risk for a gas explosion at a later stage may be imminent. Li-ion batteries release a various number of toxic substances14,15,16 as well as e.g. CO (an asphyxiant gas) and CO2 (induces anoxia) during heating and fire. At elevated temperature the fluorine content of the electrolyte and, to some extent, other parts of the battery such as the polyvinylidene fluoride (PVdF) binder in the electrodes, may form gases such as hydrogen fluoride HF, phosphorus pentafluoride (PF5) and phosphoryl fluoride (POF3).
The explosive problem with recycling iPads, iPhones and other gadgets: They literally catch fire.
Around the world, garbage trucks and recycling centers are going up in flames. The root of the problem: volatile lithium-ion batteries sealed inside our favorite electronics from Apple, Samsung, Microsoft and more. They’re not only dangerous but also difficult to take apart — making e-waste less profitable, and contributing to a growing recycling crisis...
For all their benefits at making our devices slim, powerful and easy to recharge, lithium-ion batteries have some big costs. They contain Cobalt, often mined in inhumane circumstances in places like the Congo. And when crushed, punctured, ripped or dropped, lithium-ion batteries can produce what the industry euphemistically calls a “thermal event.” It happens because these batteries short circuit when the super-thin separator between their positive and negative parts gets breached. Remember Samsung’s exploding Note 7 smartphone? That was a lithium-ion thermal event.
Old devices end up in trouble when we throw them in the trash, stick them in the recycling bin, or even responsibly bring them to an e-waste center. There isn’t official data on these fires, but the anecdotal evidence is stark. Since the spring of 2018 alone, batteries have been suspected as the cause of recycling fires in New York, Arizona, Florida, Wisconsin, Indiana, Idaho, Scotland, Australia and New Zealand. In California, a recent survey of waste management facilities found 83 percent had at least one fire over the last two years, of which 40 percent were caused by lithium-ion batteries.
In 2016, the Shoreway Environmental Center that serves Silicon Valley suffered a 4-alarm fire it suspects was caused by a lithium-ion battery that went undetected amid other junk in its sorting systems. The fire damage cost $8.5 million.
There’s plenty of blame to go around. People shouldn’t carelessly throw battery-powered electronics into the bin. (Here’s what you should do.) Local governments haven’t figured out good ways for us to hand off of this common but dangerous material. The tech press (including me) should write less about shiny new things and more about how to make old stuff last longer. Some gadget makers, including Apple, are taking steps to make recycling easier.
But ultimately, this is an environmental problem of the tech industry’s own design. And it’s time they own it.
edit on 2020927 by Arbitrageur because: clarification
I see this as a plus as other researchers will look at building bigger and better batteries of this type. Remember the first solar cells were small
and had little output and expensive.
This was back in the late 1950s and early 1960s.
Now you have major solar farms with 300+ watt solar panels that cost less than the early solar cells the had an output of less than 10 watts.
This was back in the late 1950s and early 1960s.
Now you have major solar farms with 300+ watt solar panels that cost less than the early solar cells the had an output of less than 10 watts.
new topics
-
Remember These Attacks When President Trump 2.0 Vengeance-Retribution Commences.
2024 Elections: 18 seconds ago -
Predicting The Future: The Satanic Temple v. Florida
Conspiracies in Religions: 8 minutes ago -
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies: 2 hours ago -
Hurt my hip; should I go see a Doctor
General Chit Chat: 3 hours ago -
Israel attacking Iran again.
Middle East Issues: 4 hours ago -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 4 hours ago -
When an Angel gets his or her wings
Religion, Faith, And Theology: 5 hours ago -
Comparing the theology of Paul and Hebrews
Religion, Faith, And Theology: 5 hours ago -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 6 hours ago -
Boston Dynamics say Farewell to Atlas
Science & Technology: 7 hours ago
top topics
-
The Democrats Take Control the House - Look what happened while you were sleeping
US Political Madness: 10 hours ago, 18 flags -
In an Historic First, In N Out Burger Permanently Closes a Location
Mainstream News: 12 hours ago, 16 flags -
A man of the people
Medical Issues & Conspiracies: 17 hours ago, 11 flags -
Man sets himself on fire outside Donald Trump trial
Mainstream News: 9 hours ago, 9 flags -
Biden says little kids flip him the bird all the time.
Politicians & People: 9 hours ago, 9 flags -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 6 hours ago, 6 flags -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 4 hours ago, 6 flags -
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies: 2 hours ago, 6 flags -
Israel attacking Iran again.
Middle East Issues: 4 hours ago, 5 flags -
Boston Dynamics say Farewell to Atlas
Science & Technology: 7 hours ago, 4 flags
active topics
-
Remember These Attacks When President Trump 2.0 Vengeance-Retribution Commences.
2024 Elections • 0 • : WeMustCare -
The New, New ATS Members Photos thread. Part 3.
Members • 1653 • : zosimov -
Predicting The Future: The Satanic Temple v. Florida
Conspiracies in Religions • 0 • : Degradation33 -
A man of the people
Medical Issues & Conspiracies • 15 • : PrivateAngel -
Israel attacking Iran again.
Middle East Issues • 27 • : KrustyKrab -
I hate dreaming
Rant • 7 • : TheMichiganSwampBuck -
MULTIPLE SKYMASTER MESSAGES GOING OUT
World War Three • 53 • : Zaphod58 -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media • 8 • : TheMichiganSwampBuck -
I Guess Cloud Seeding Works
Fragile Earth • 29 • : Justoneman -
Man sets himself on fire outside Donald Trump trial
Mainstream News • 40 • : Vermilion
