It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
new RCT released today on
1. PREvention
2. Early Treatment
3. Hospitalised
Take not and spread the word 1st politician to back it becomes a hero. Lets find 1
1. PREvention
2. Early Treatment
3. Hospitalised
Take not and spread the word 1st politician to back it becomes a hero. Lets find 1
~relevant side-bar~
Reuters
finance.yahoo.com...
the ? 10 toes of Statue...or else the ? 10 horns on Beast Empire in Revelation
Reuters
"Ten COVID-19 vaccines seen by mid-year, head of global pharma group says"
finance.yahoo.com...
the ? 10 toes of Statue...or else the ? 10 horns on Beast Empire in Revelation
President Trump says the first covid-19 vaccines will start being distributed in the US immediately after the FDA approval, on December 11th.
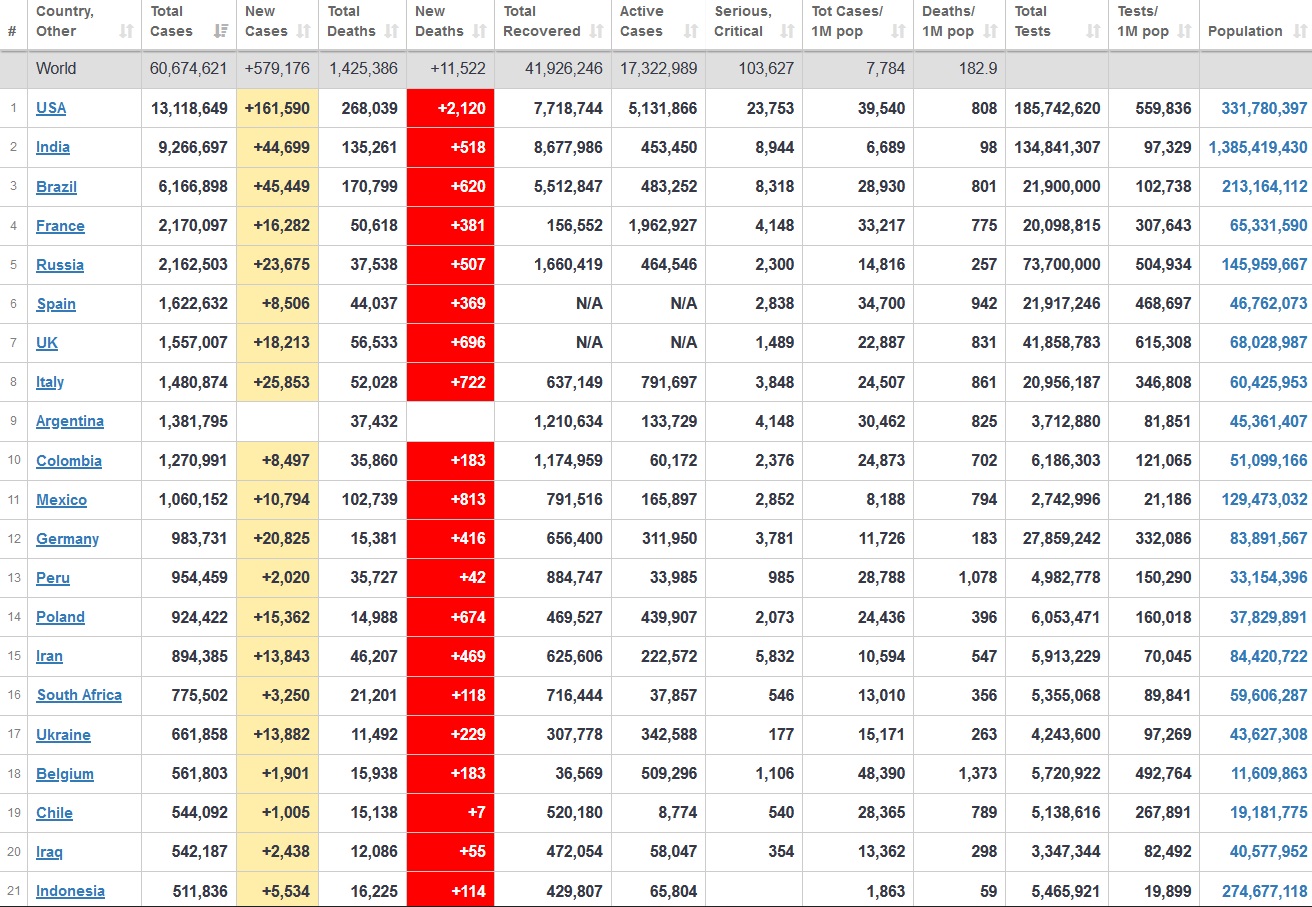
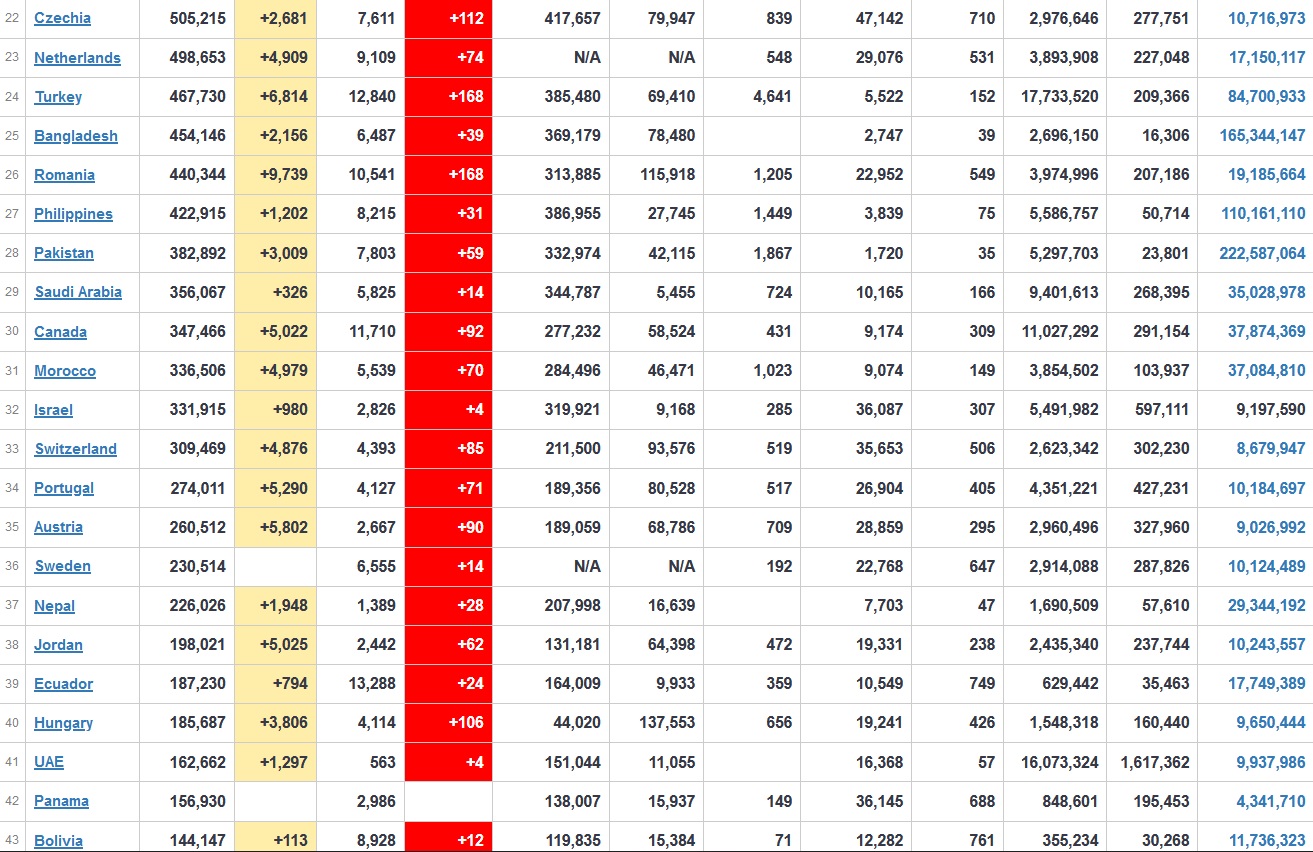
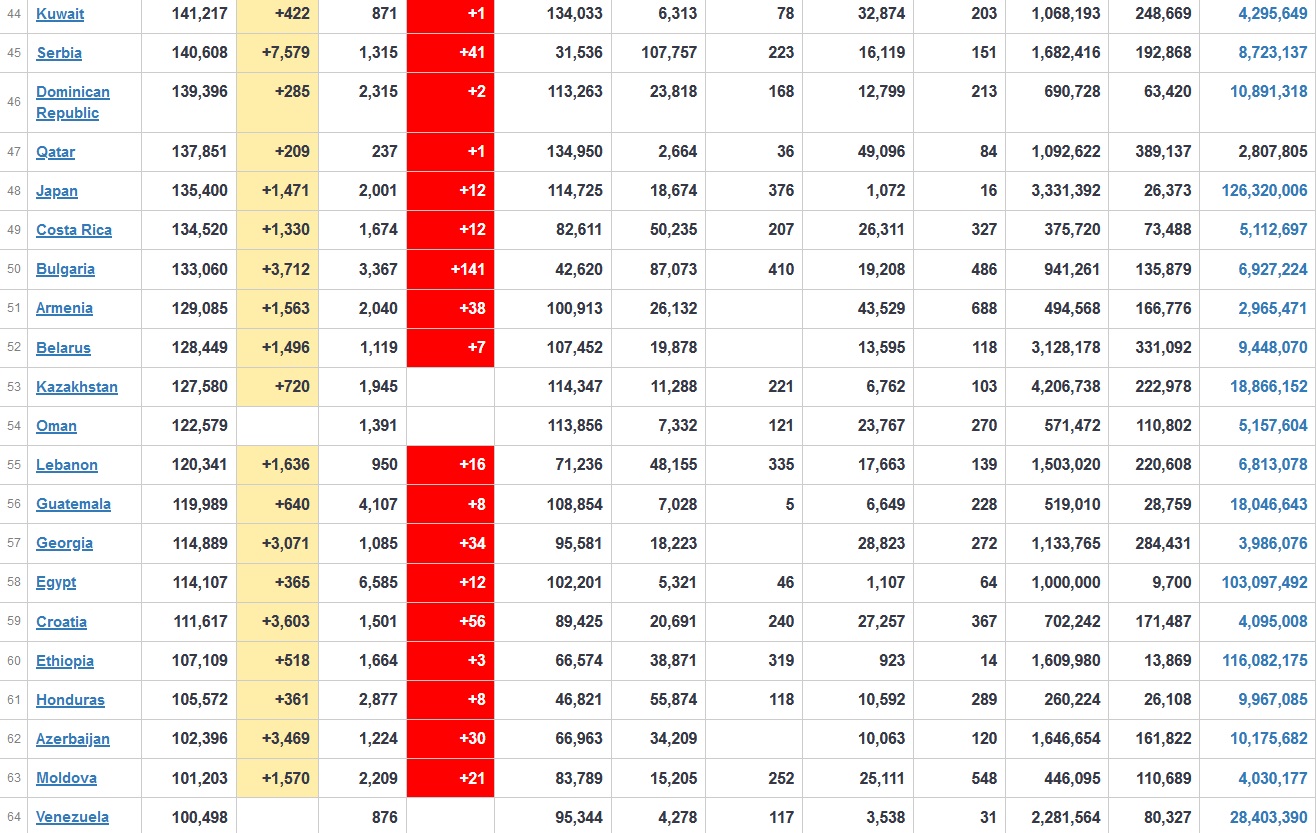
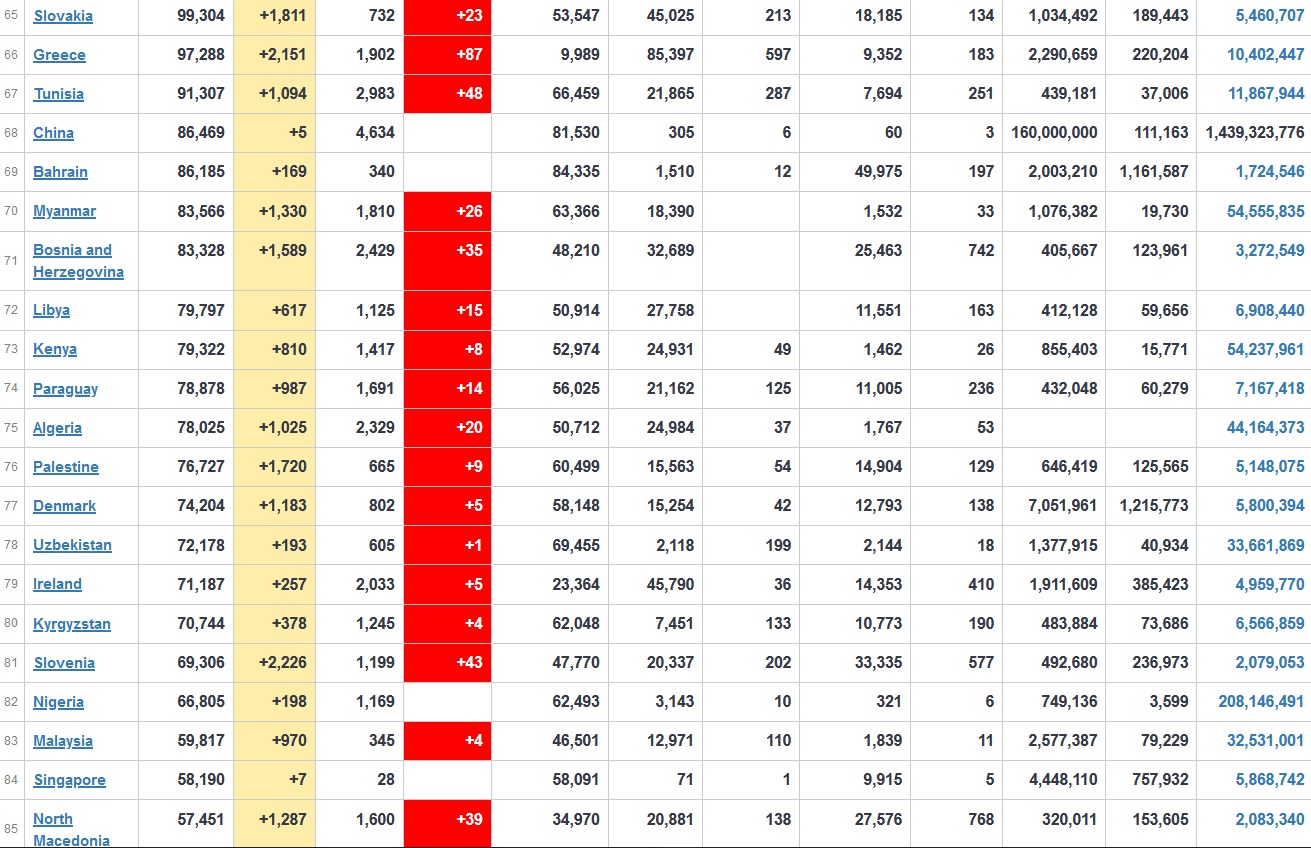
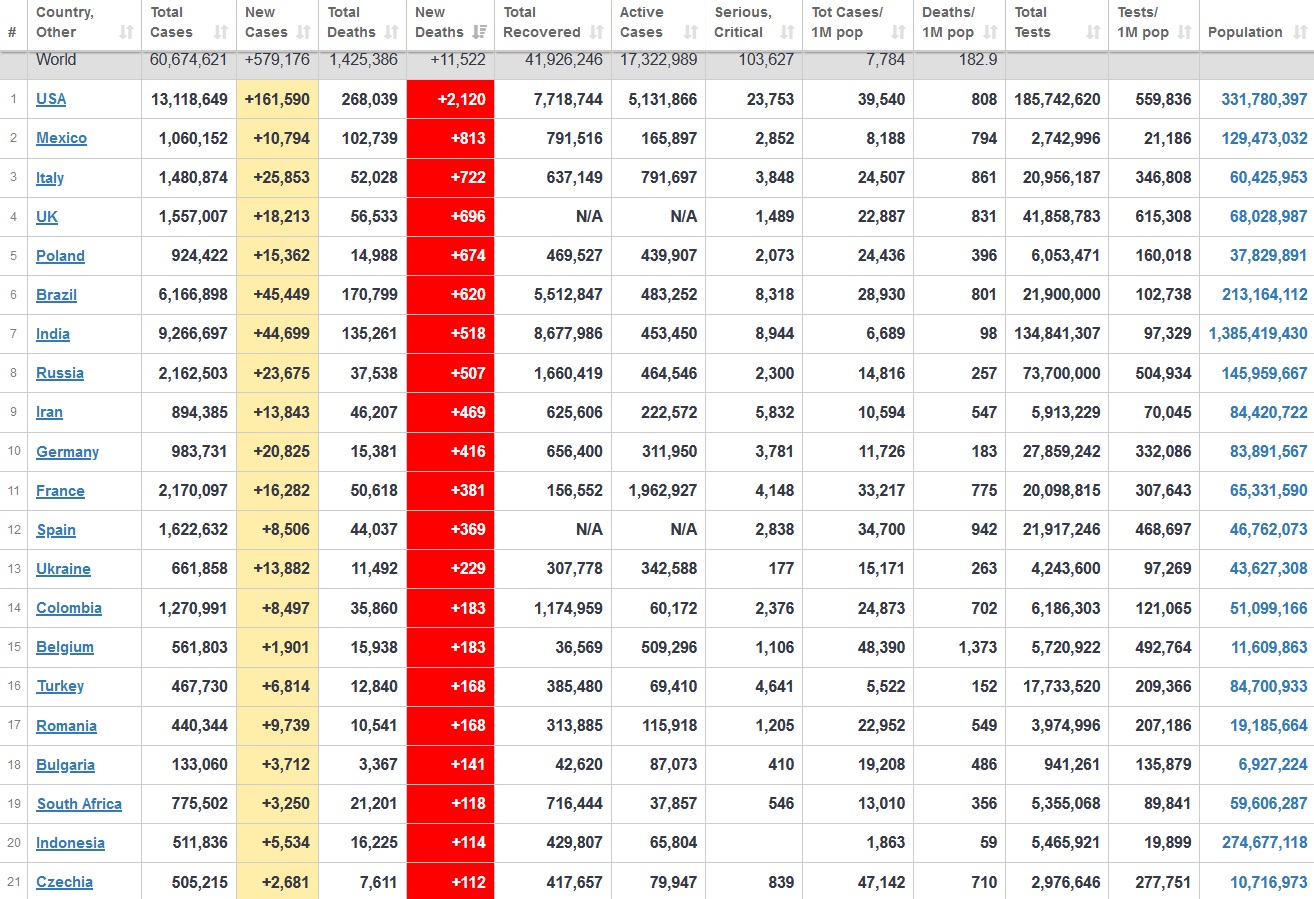
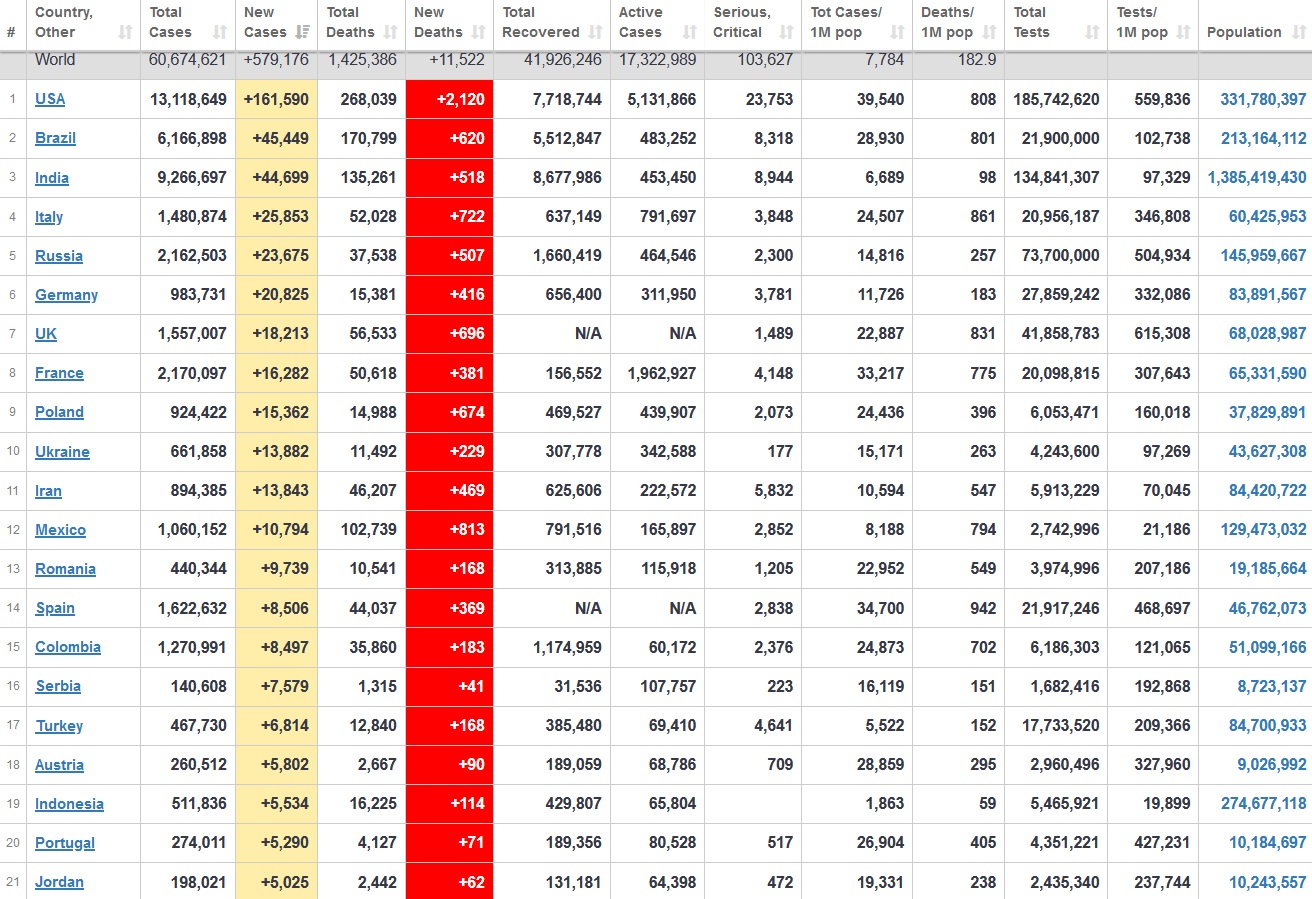
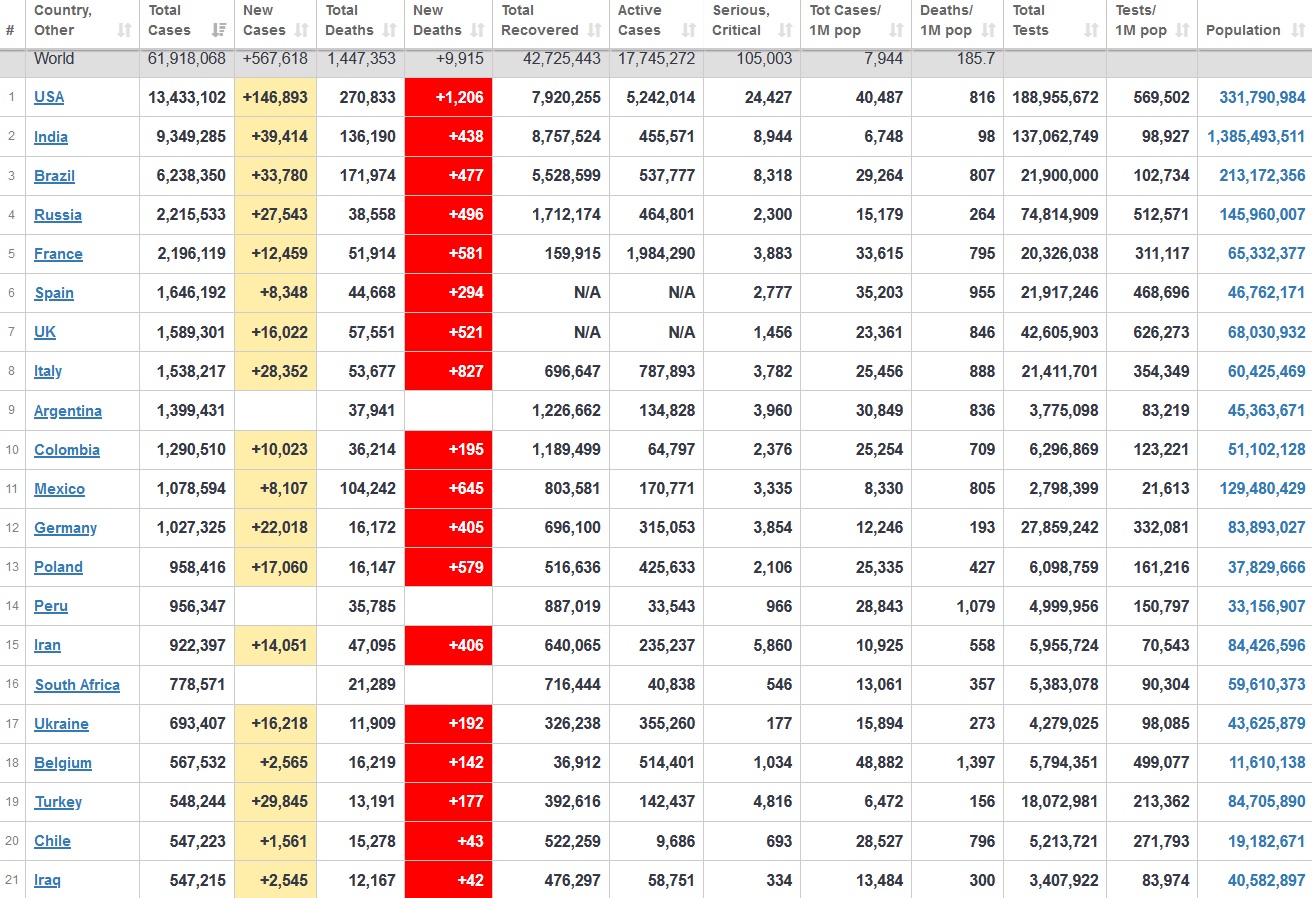
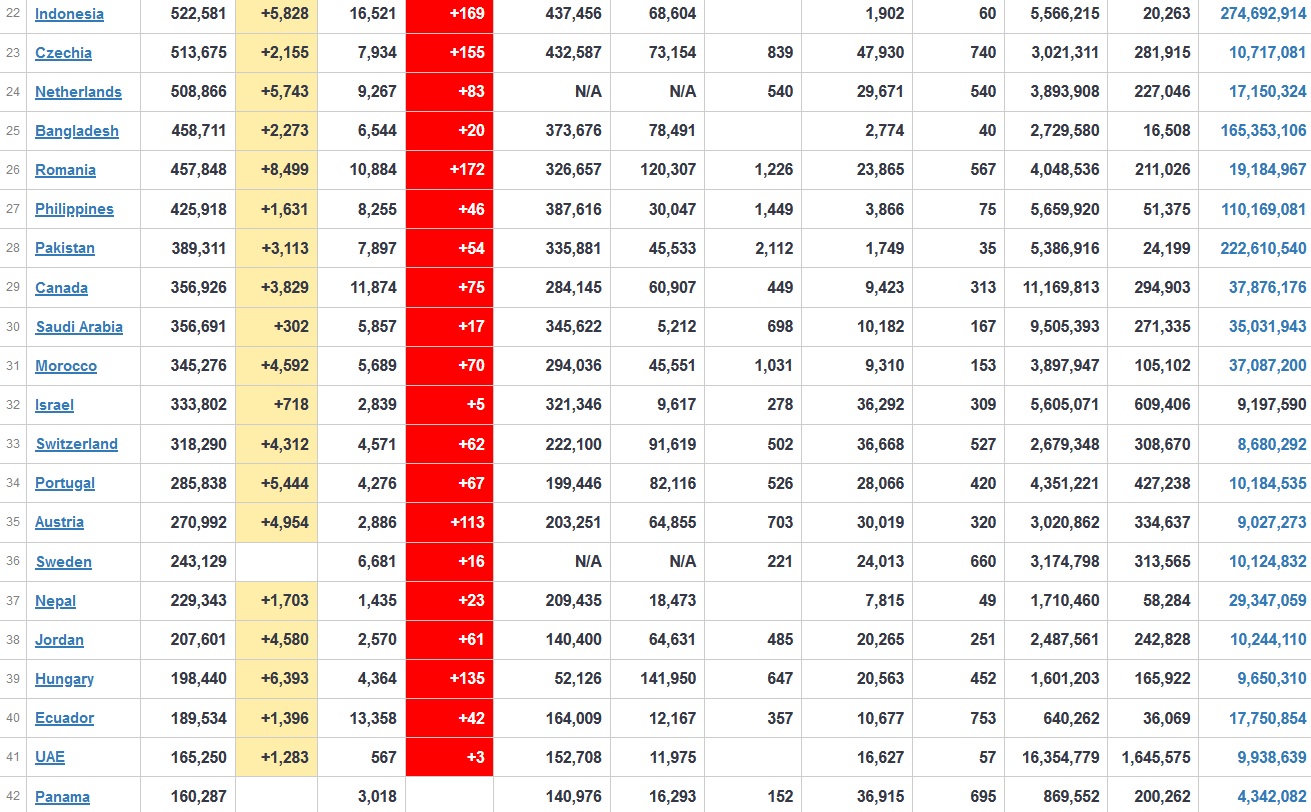
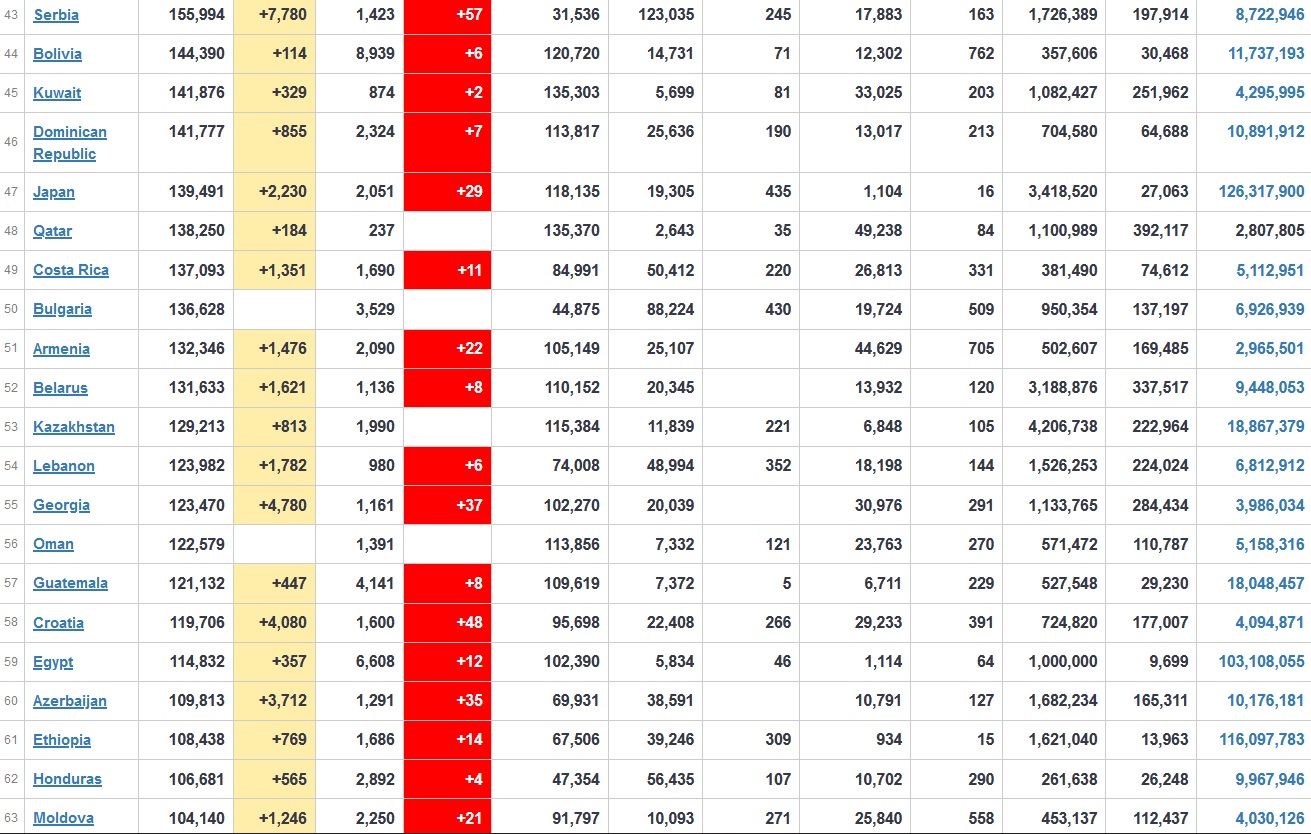
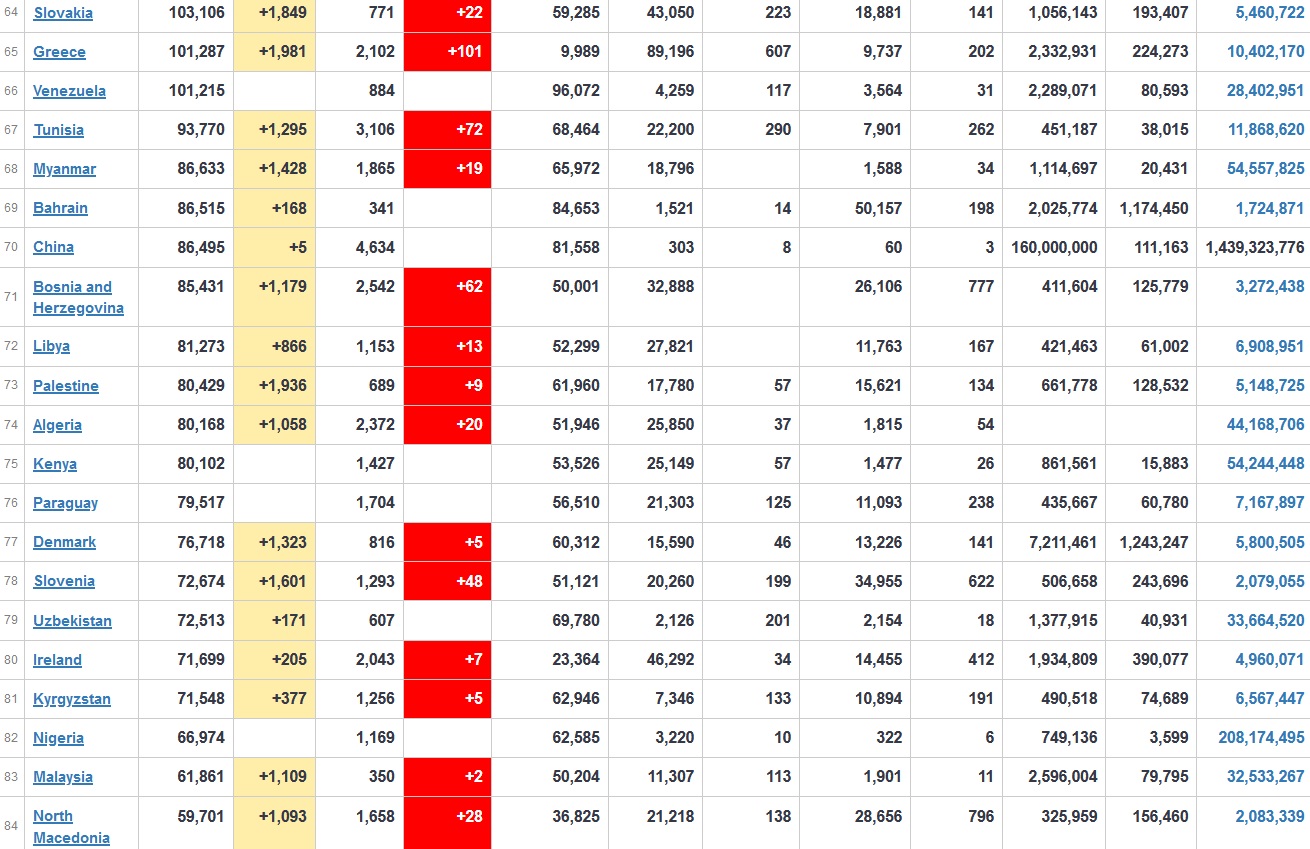
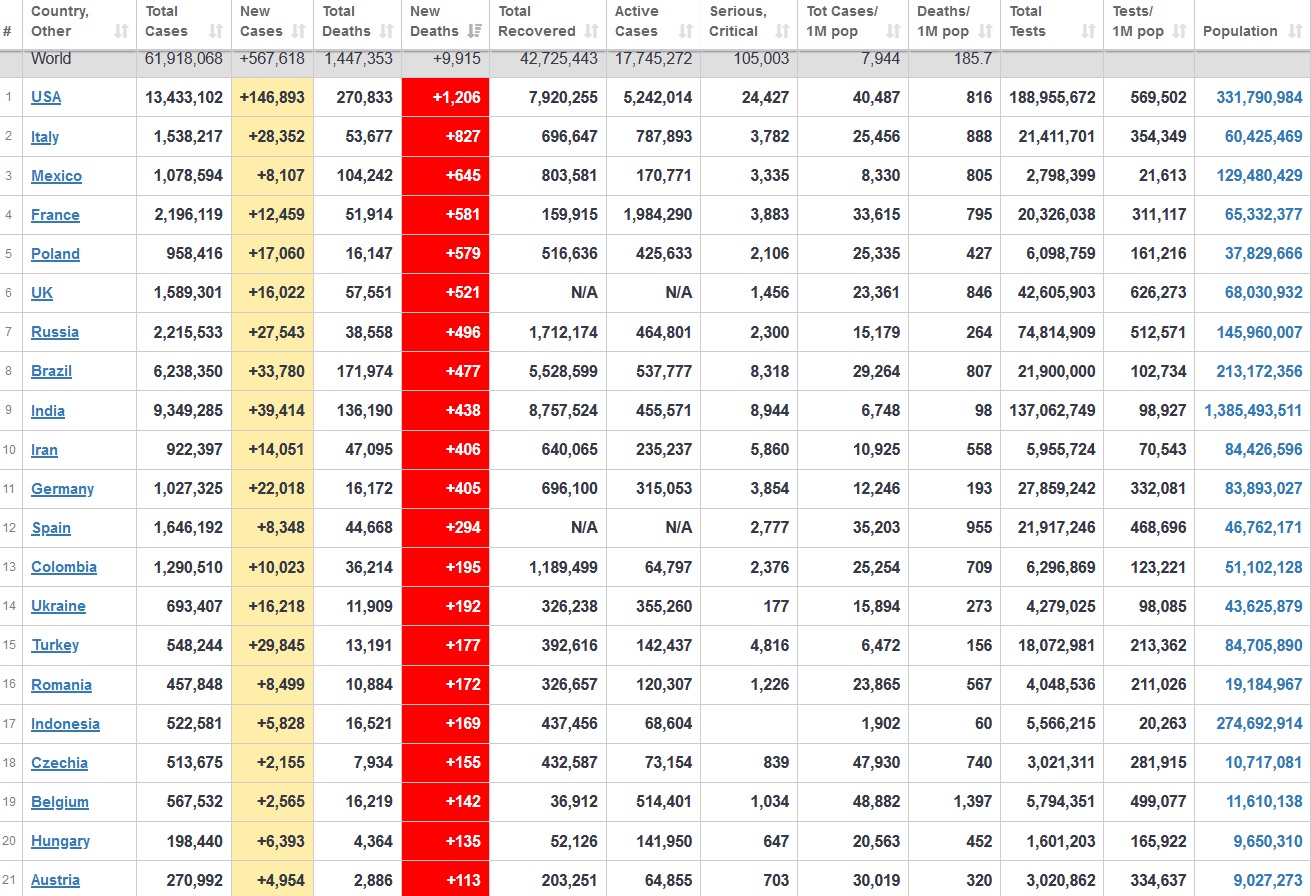
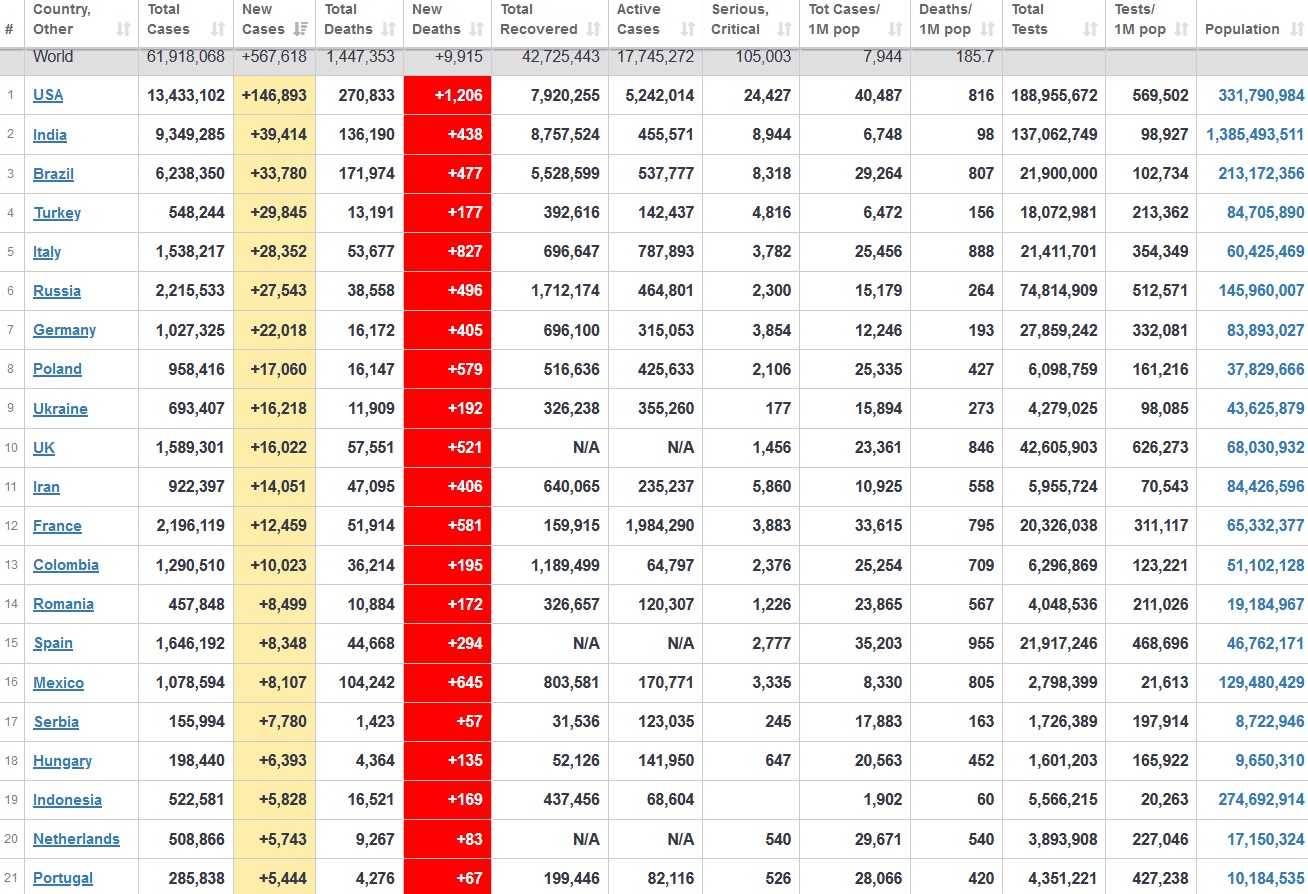
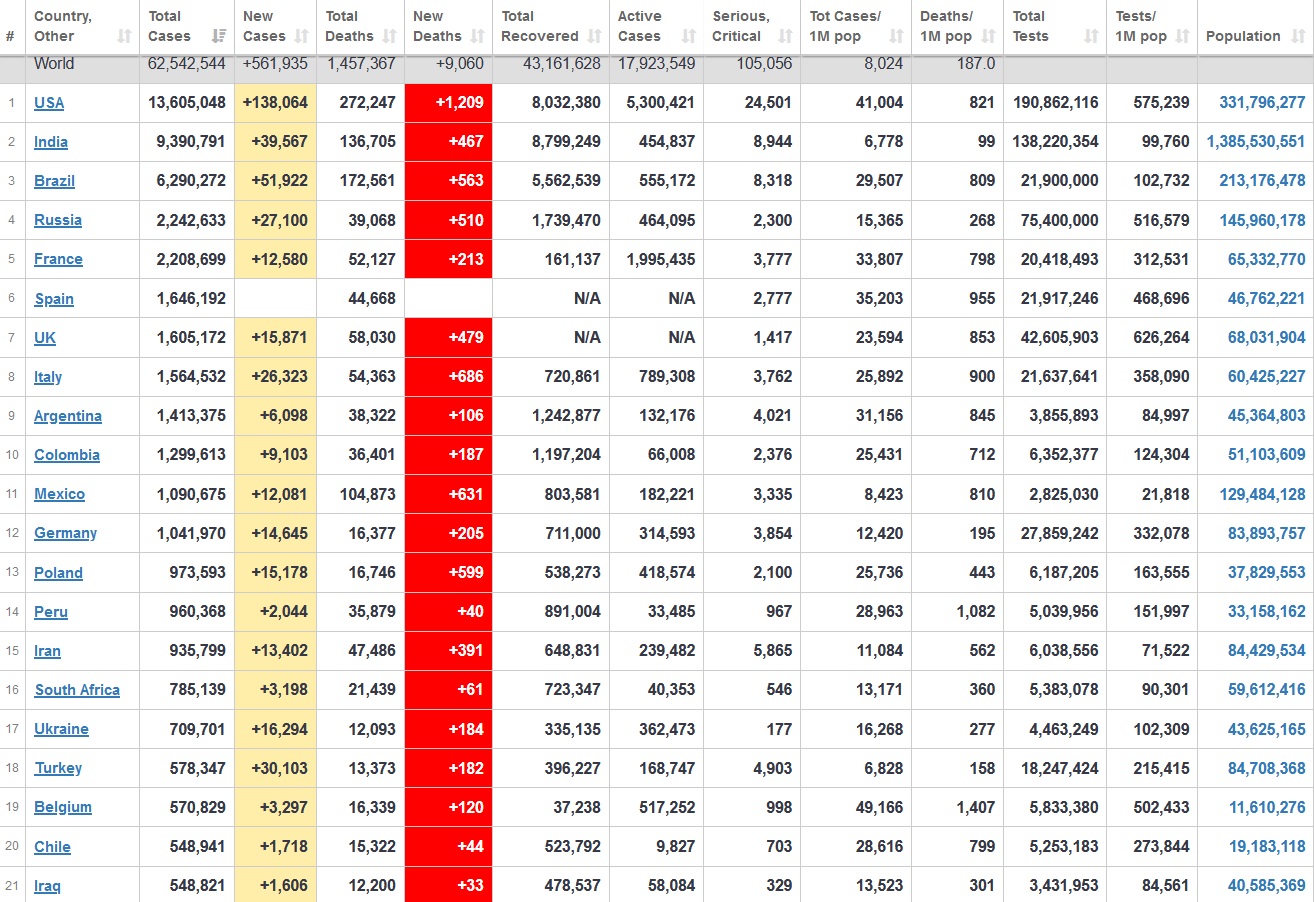
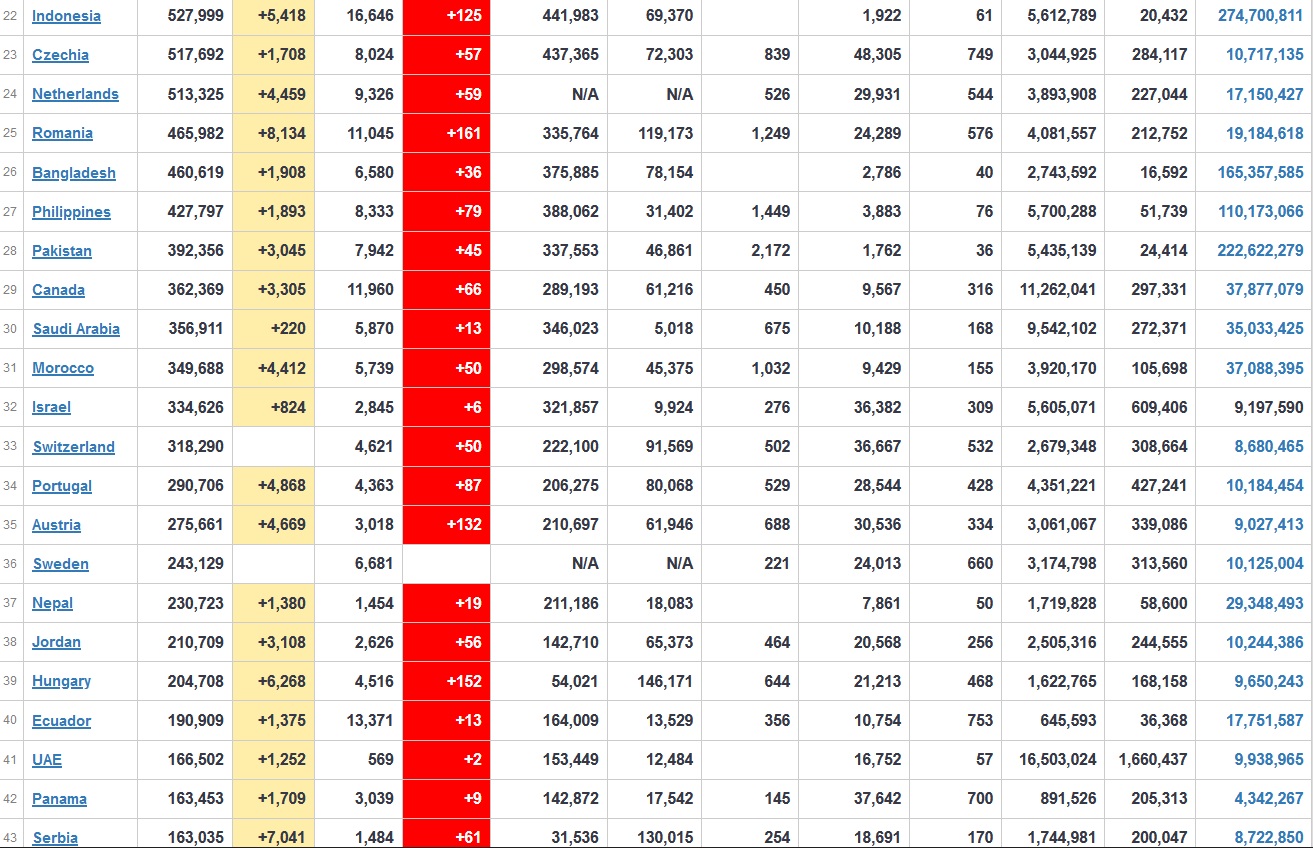
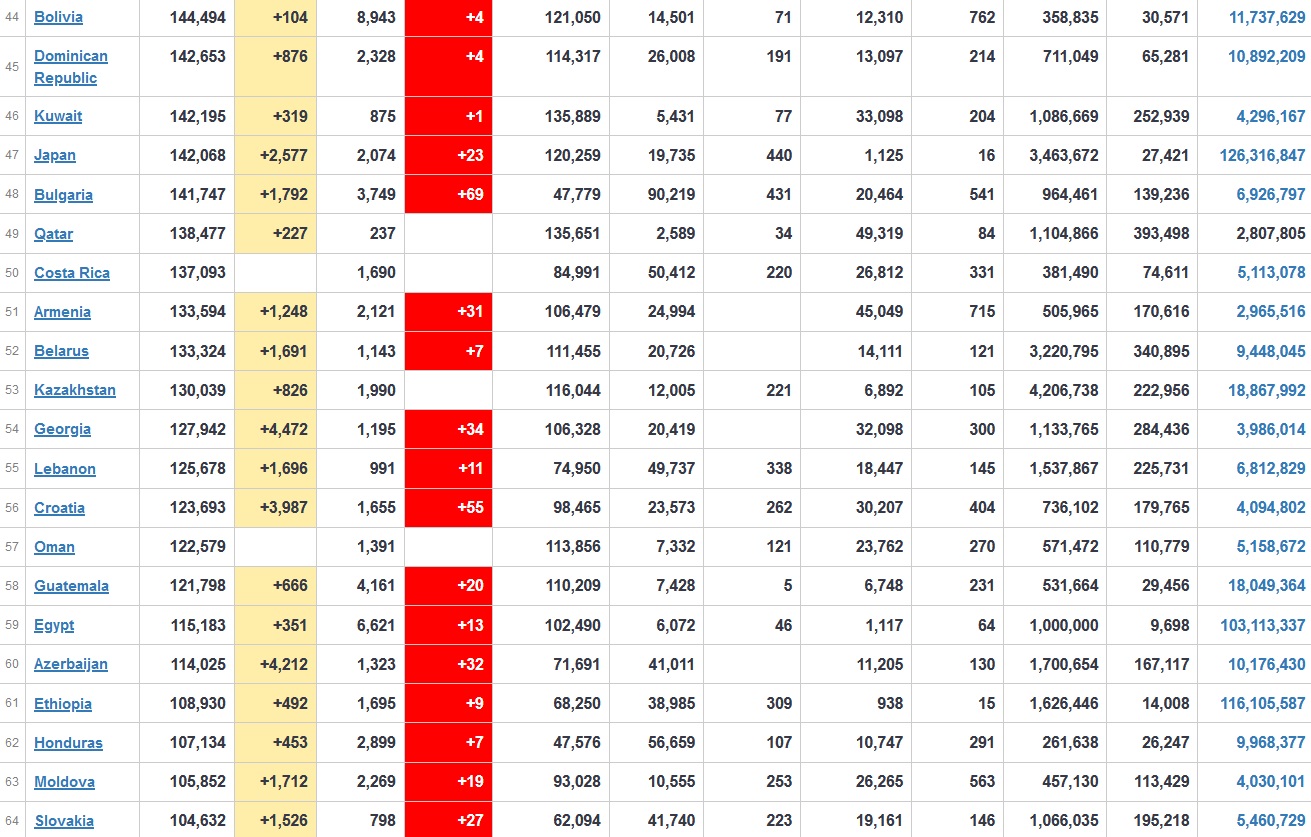
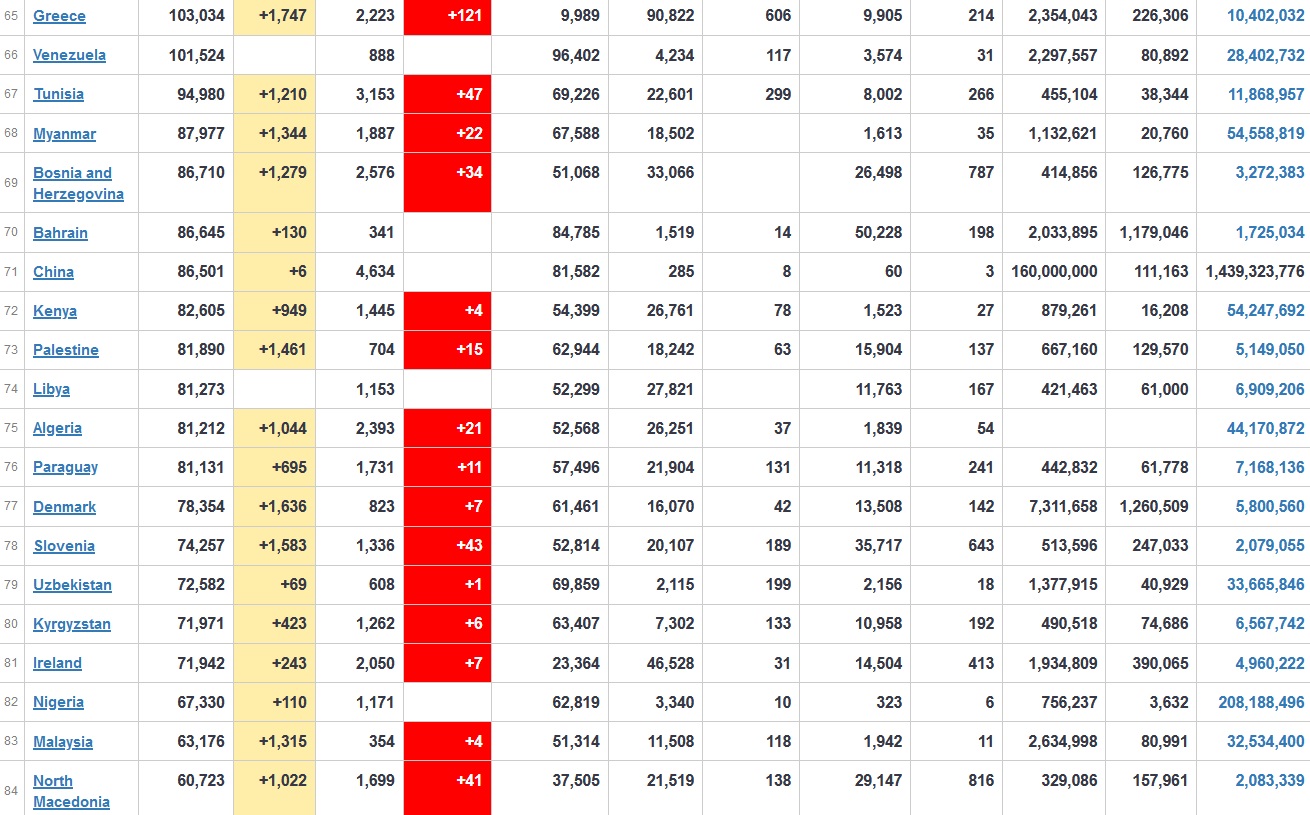
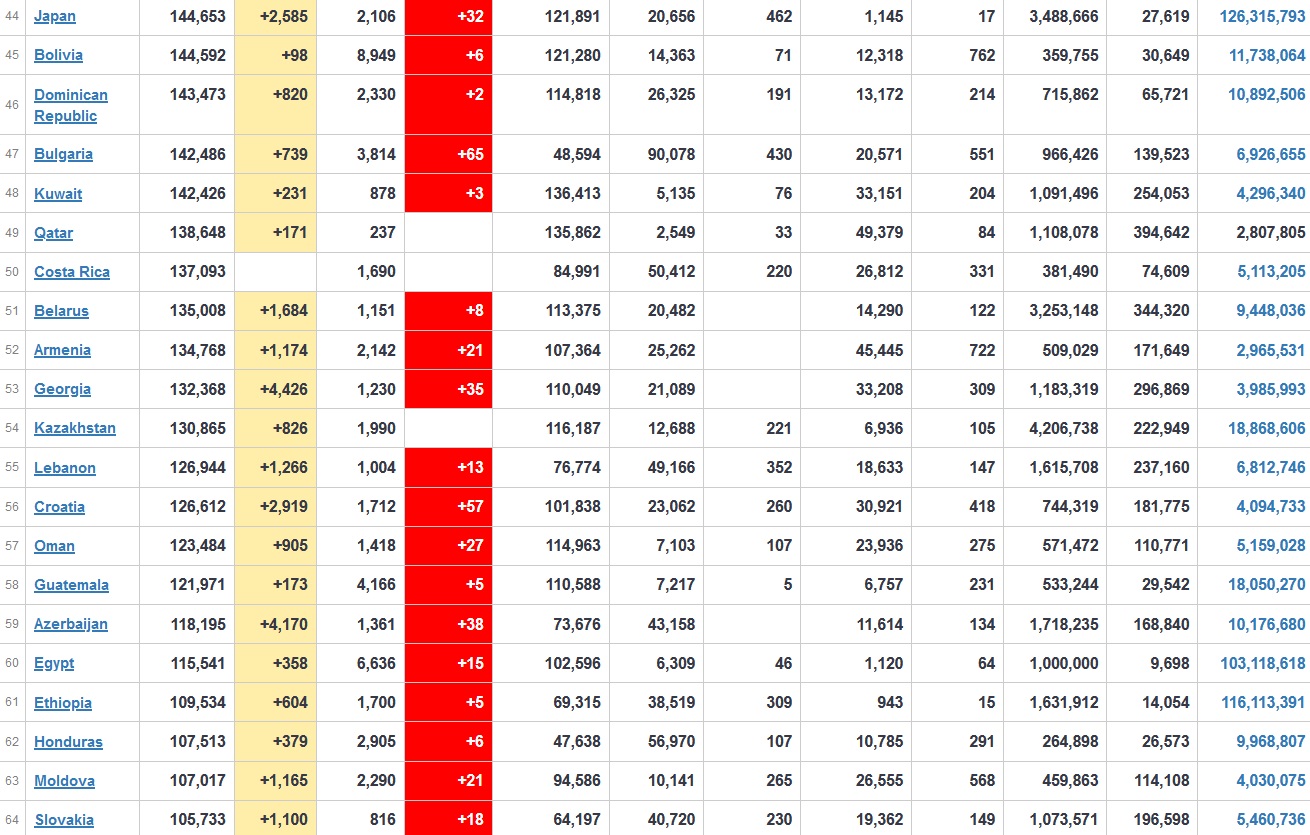
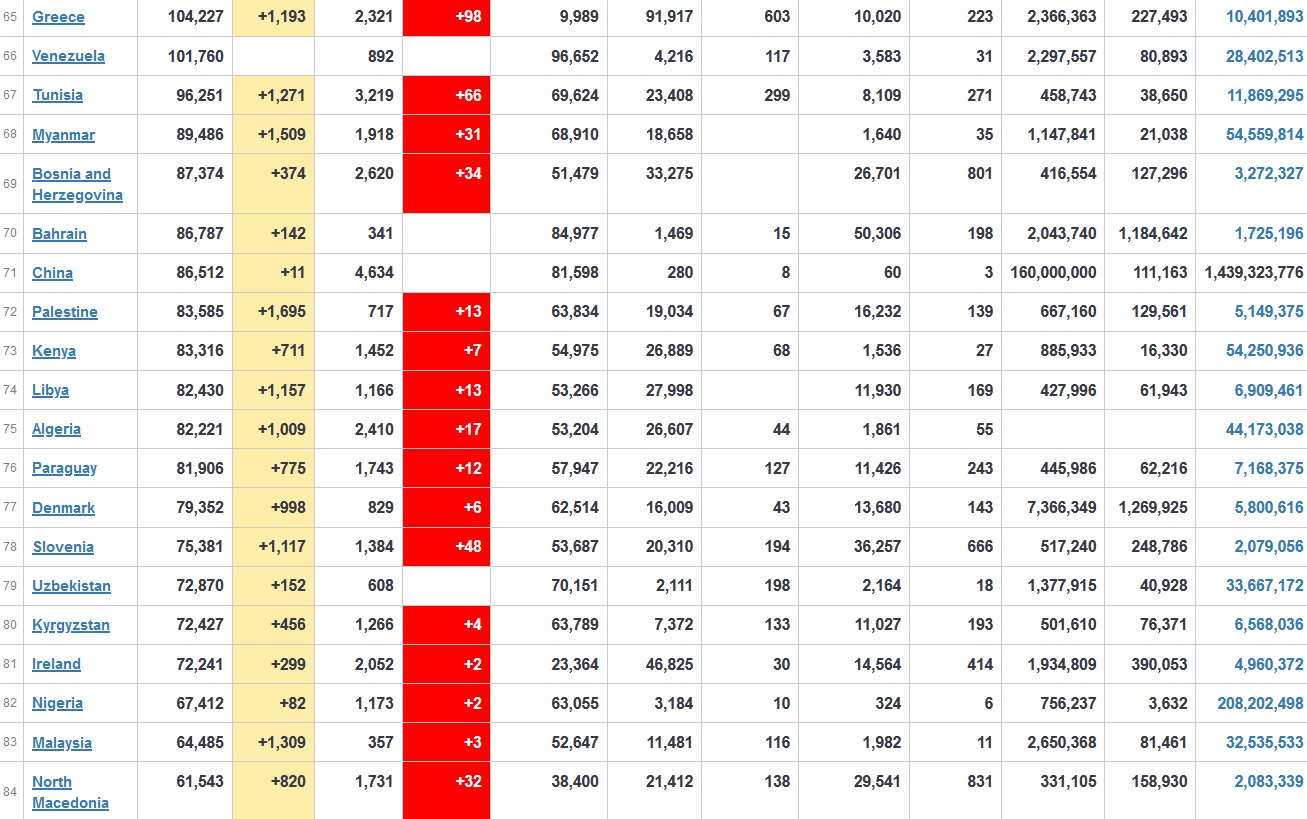
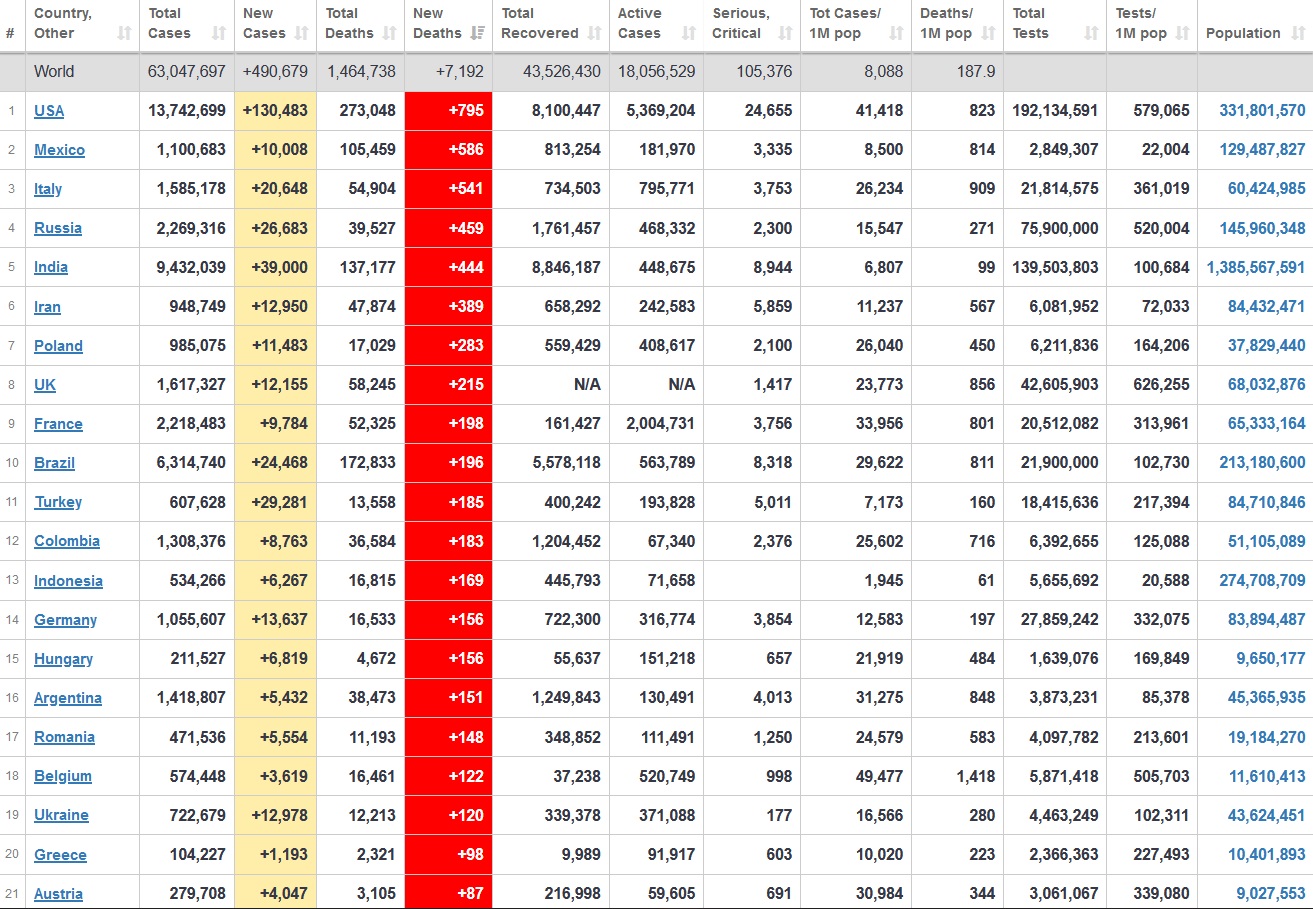
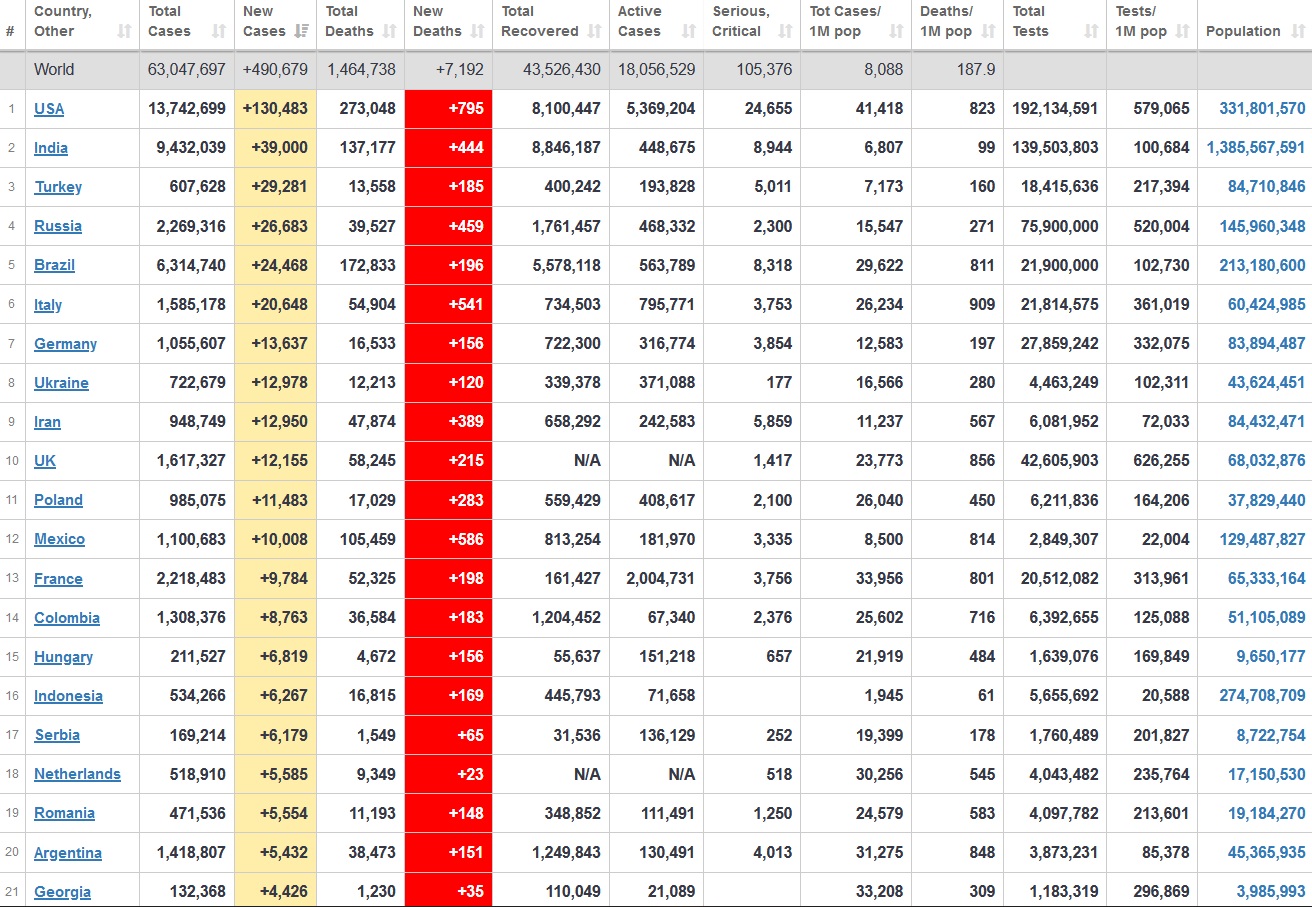
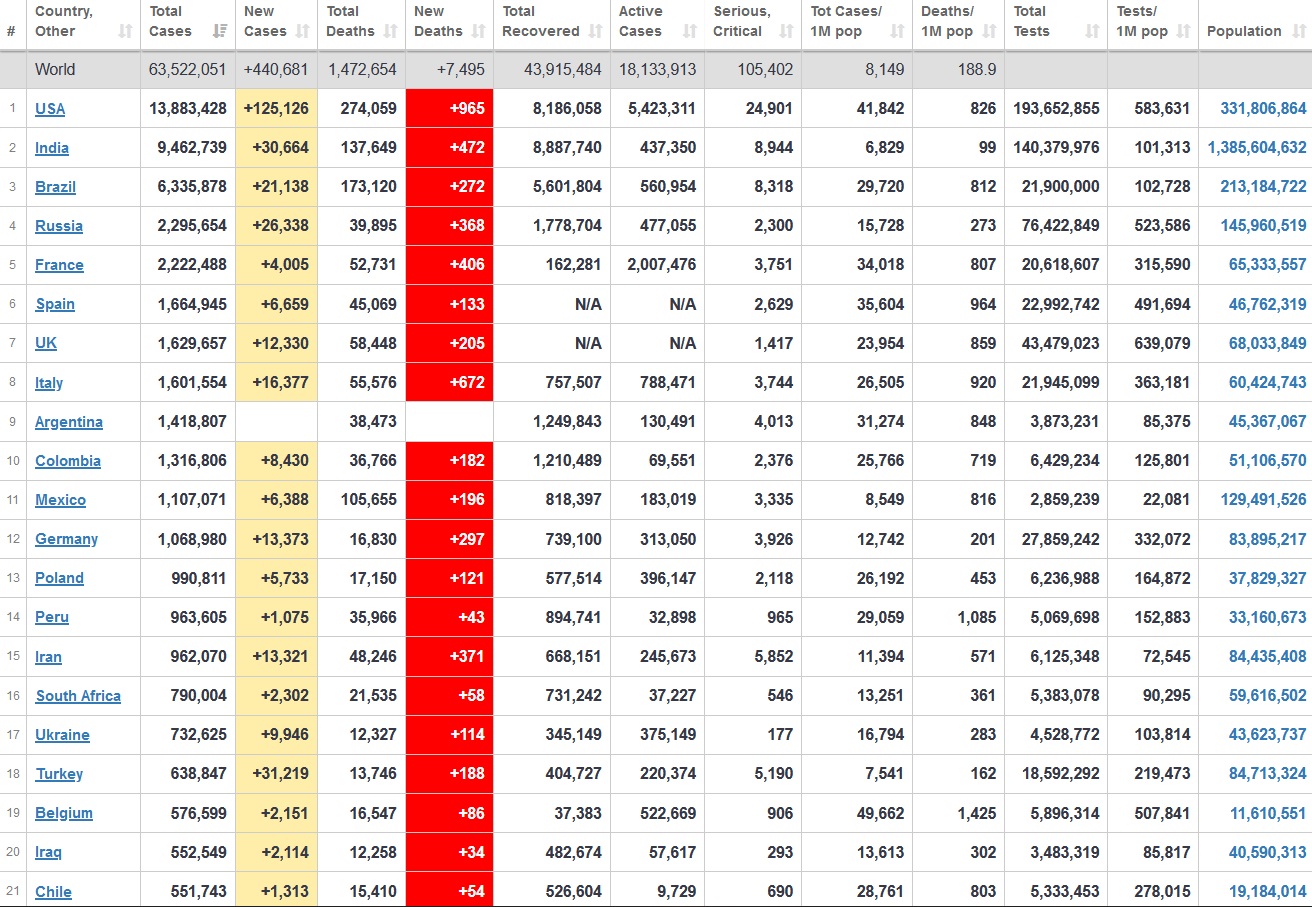
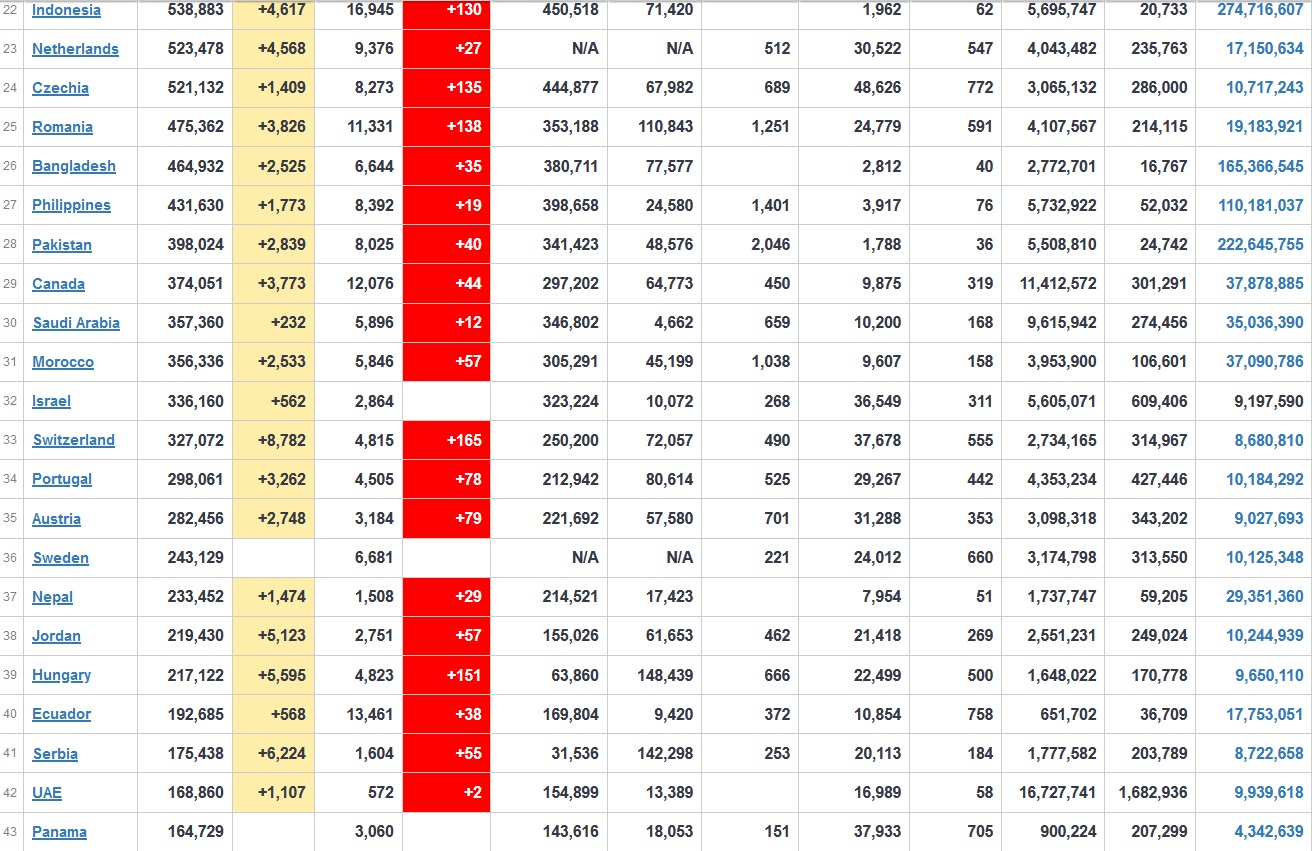
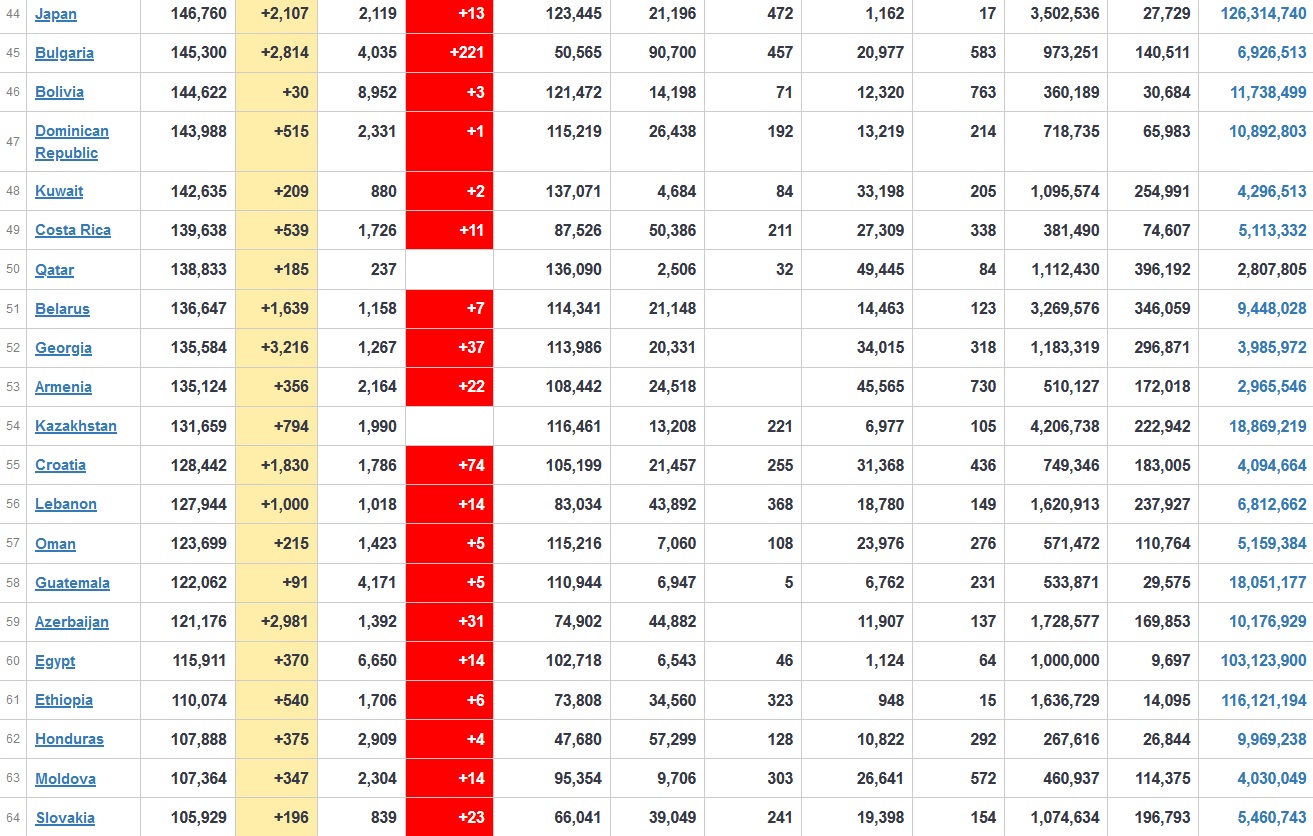
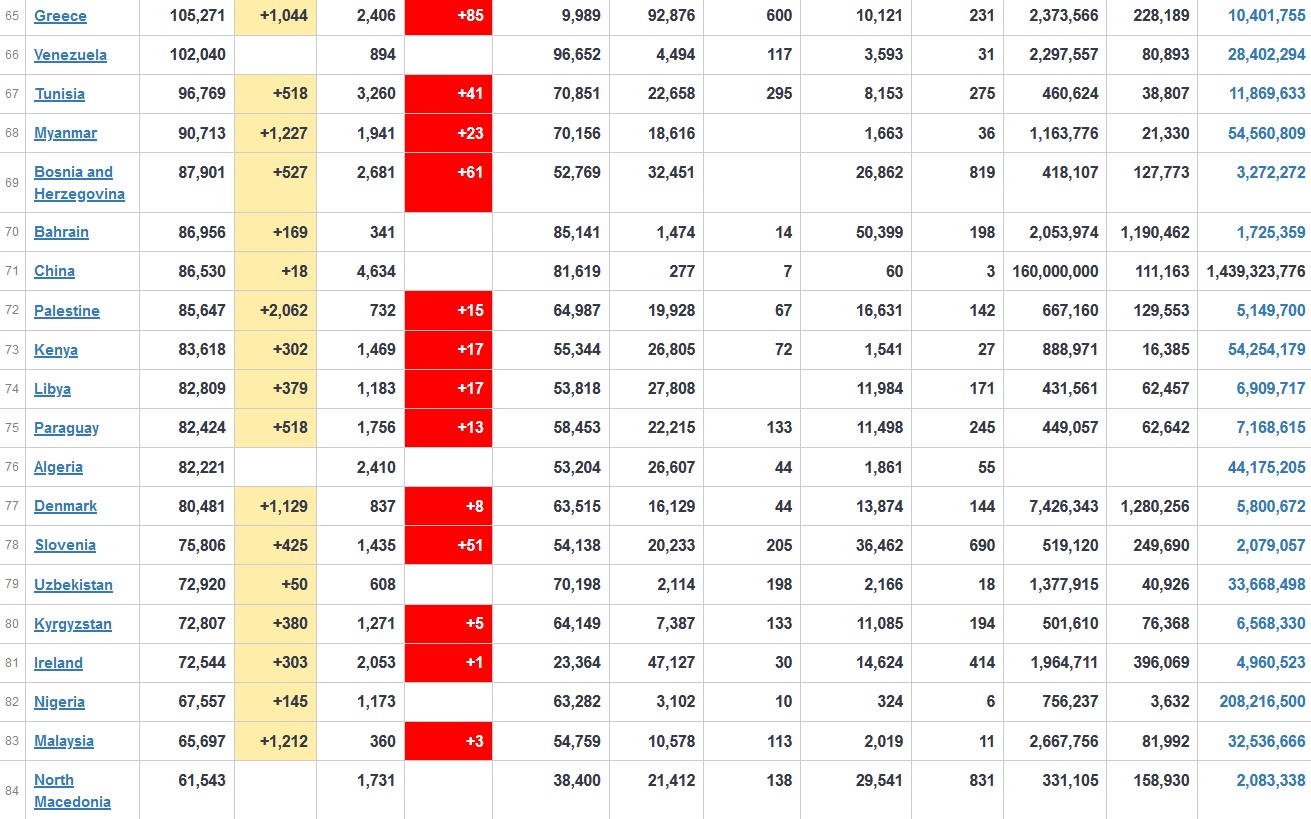
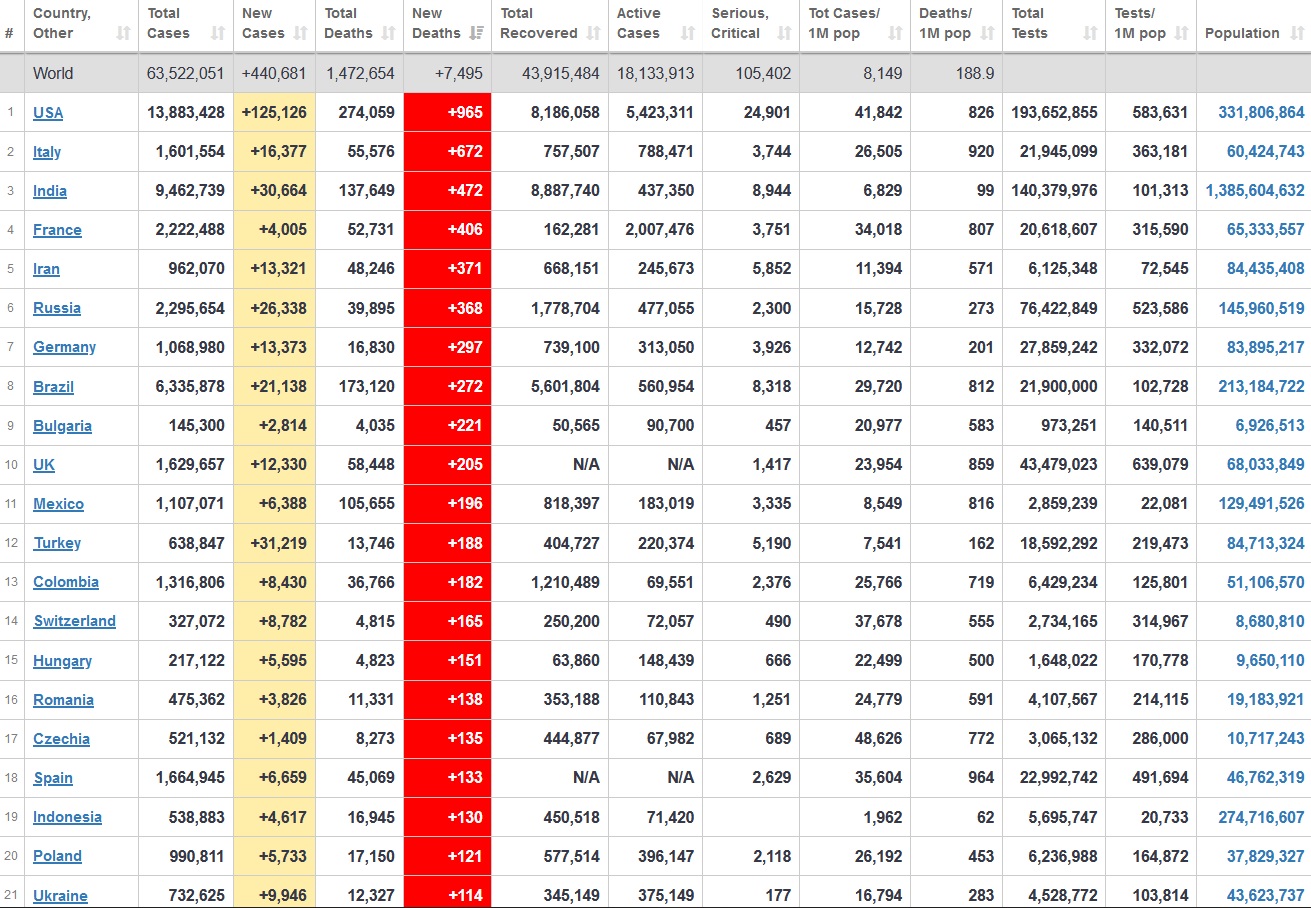
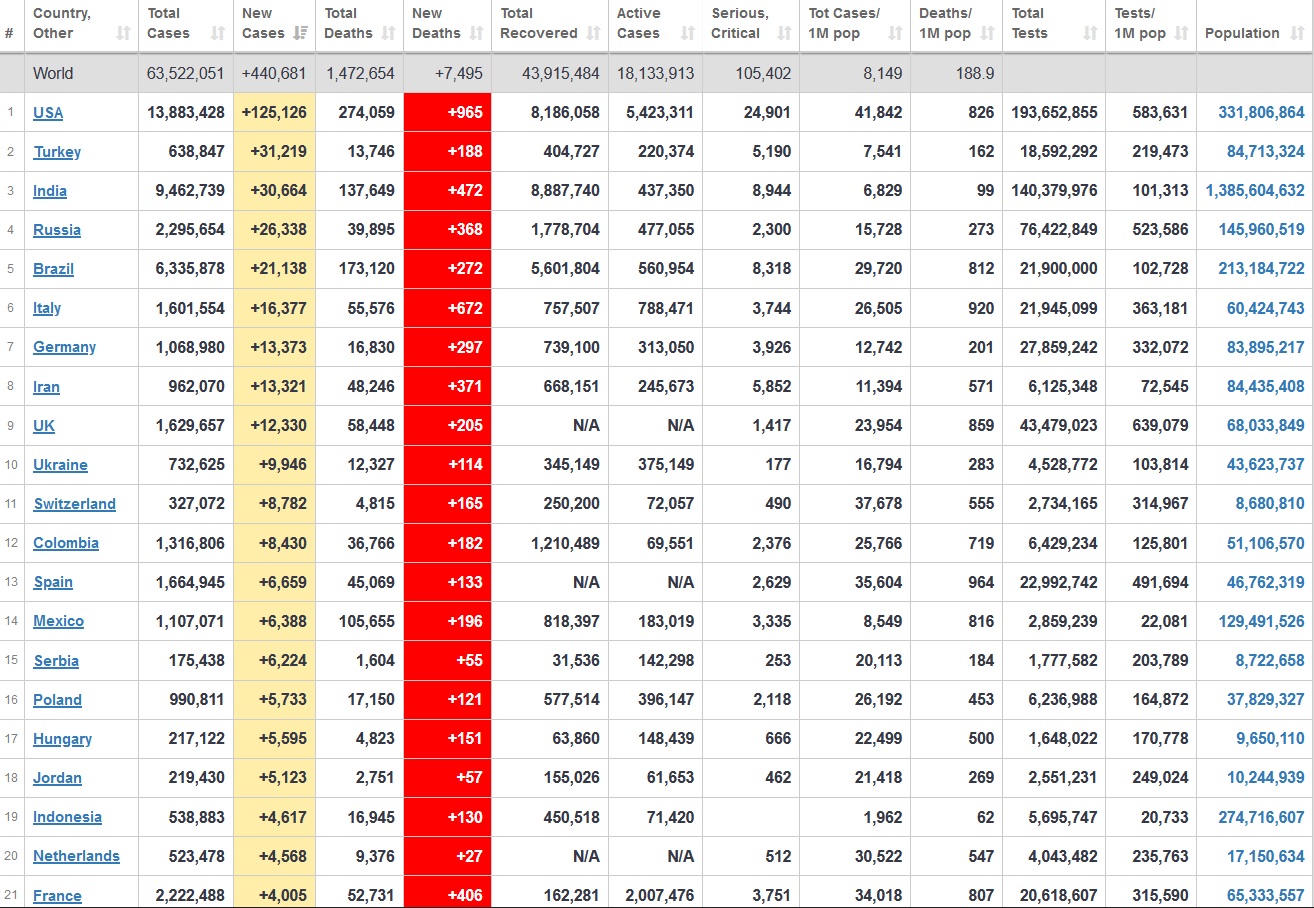
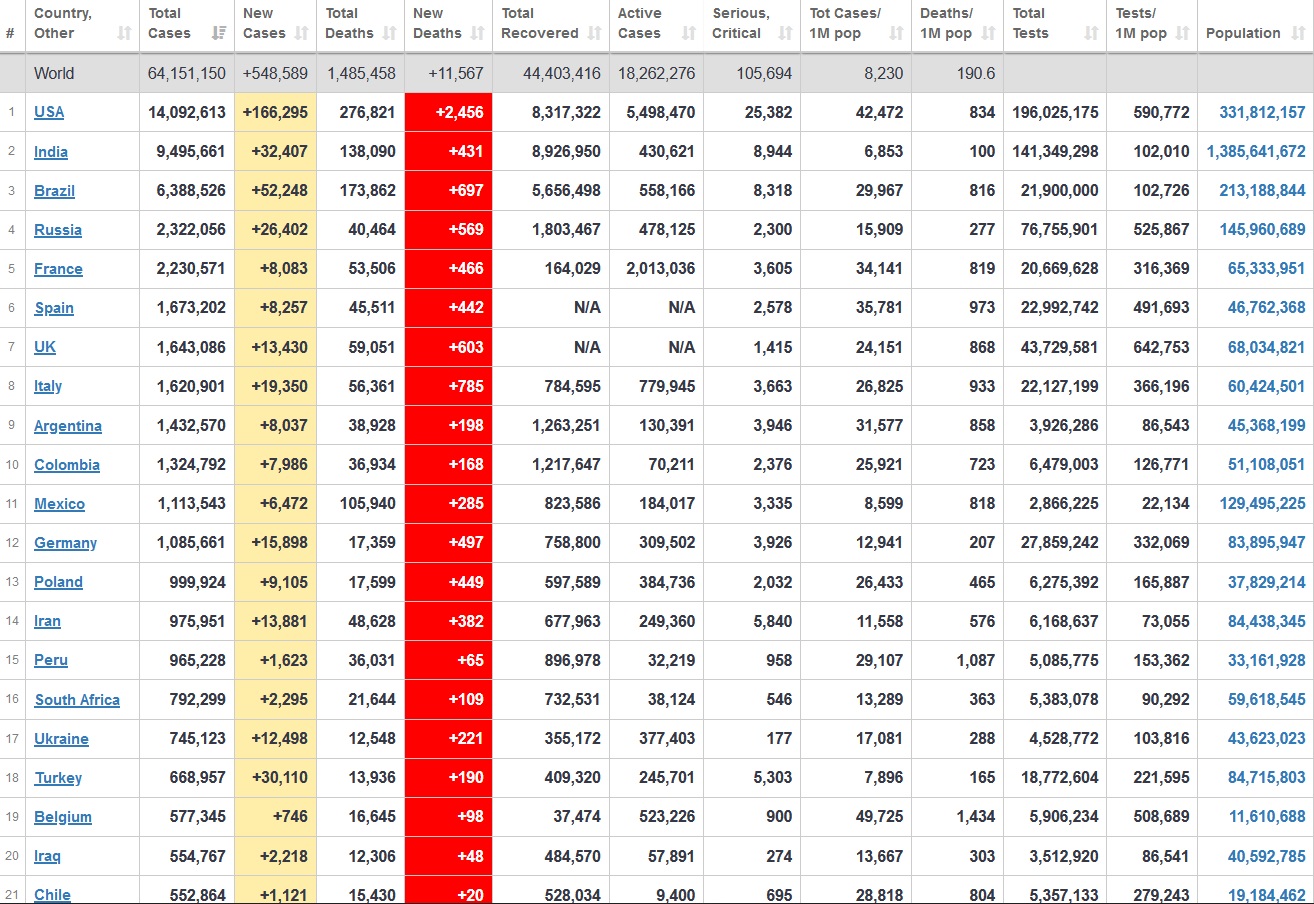
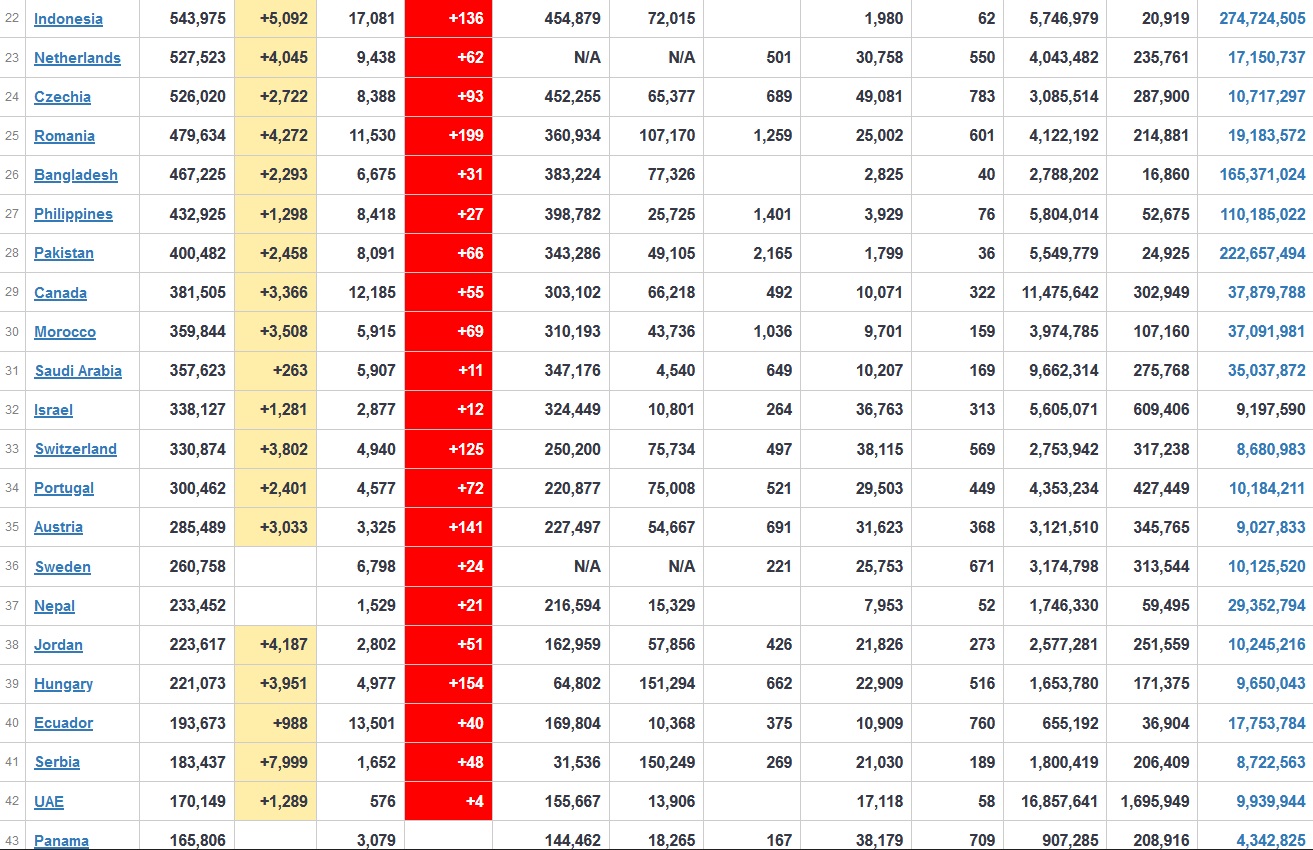
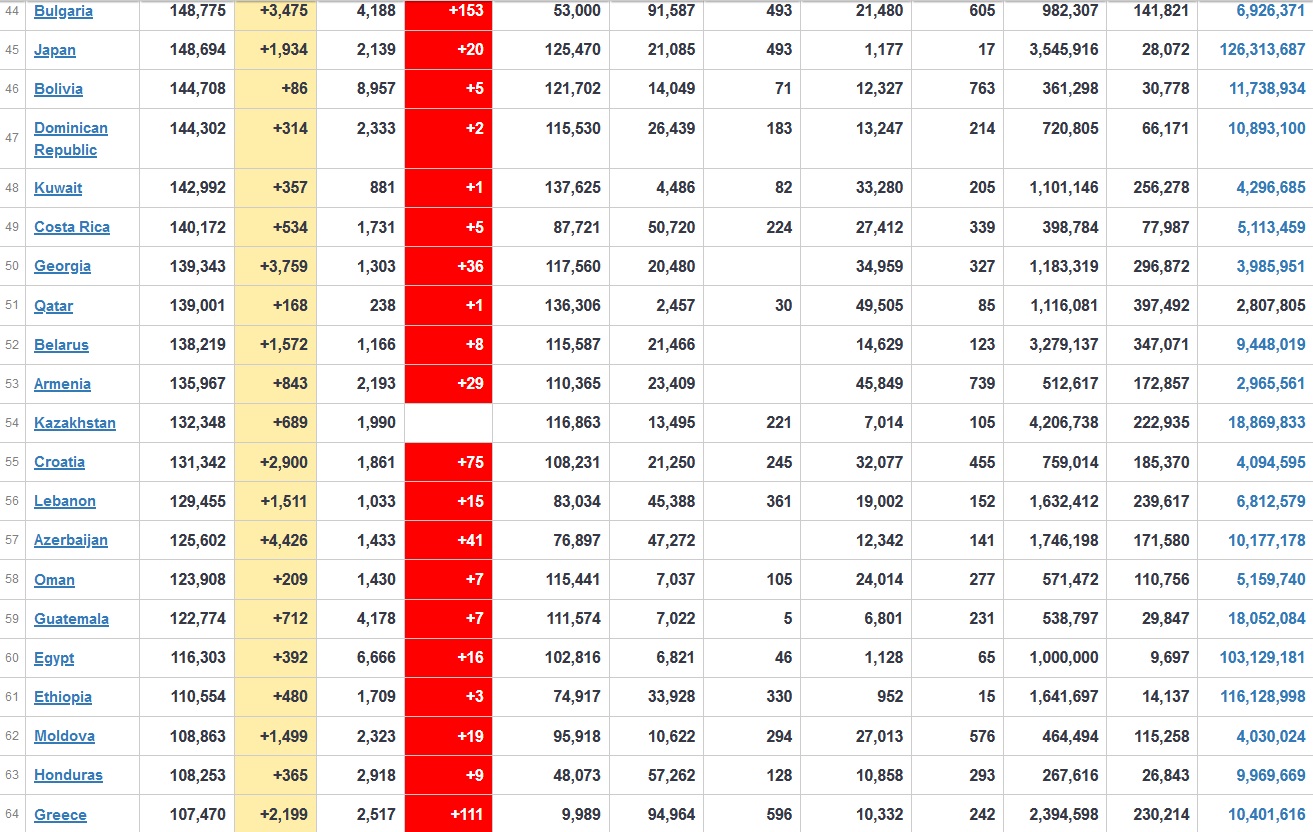
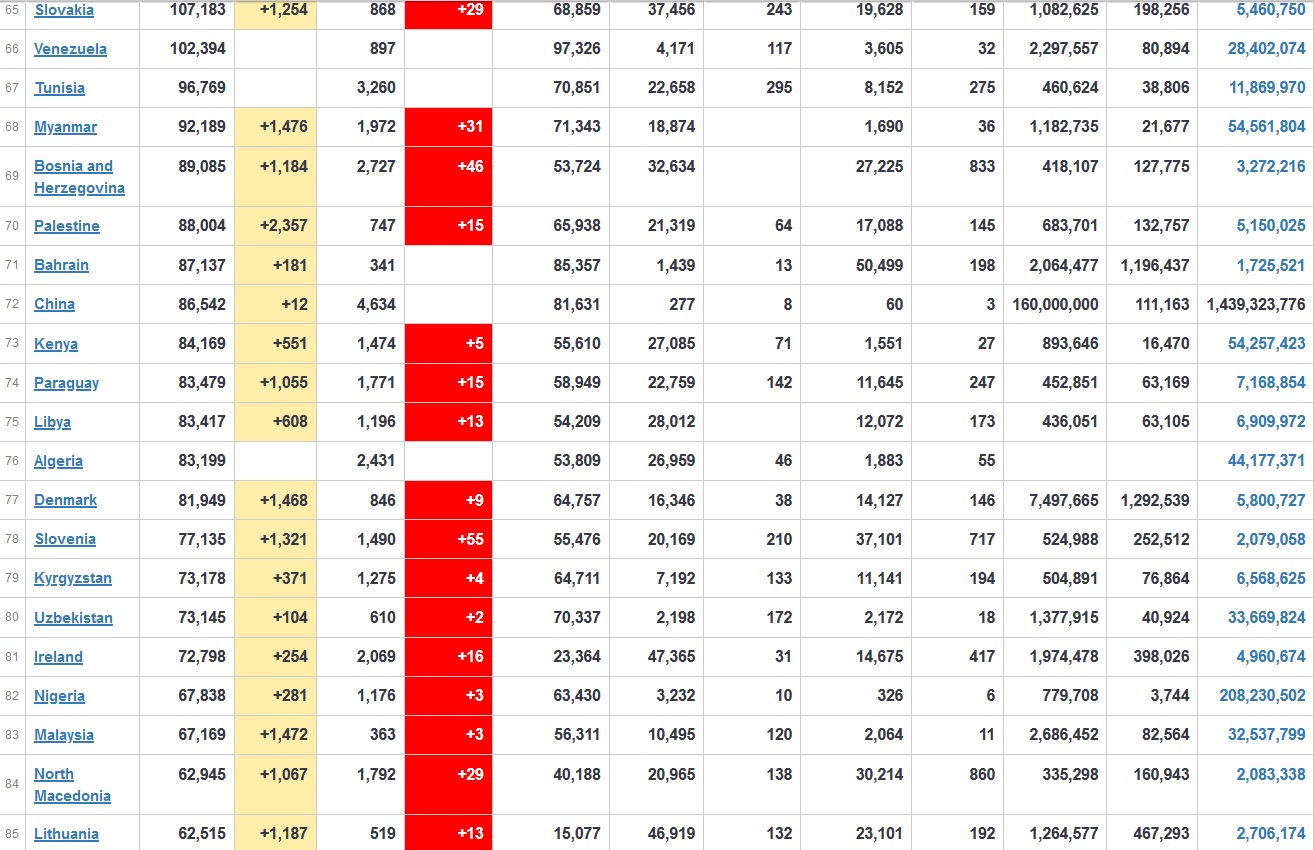
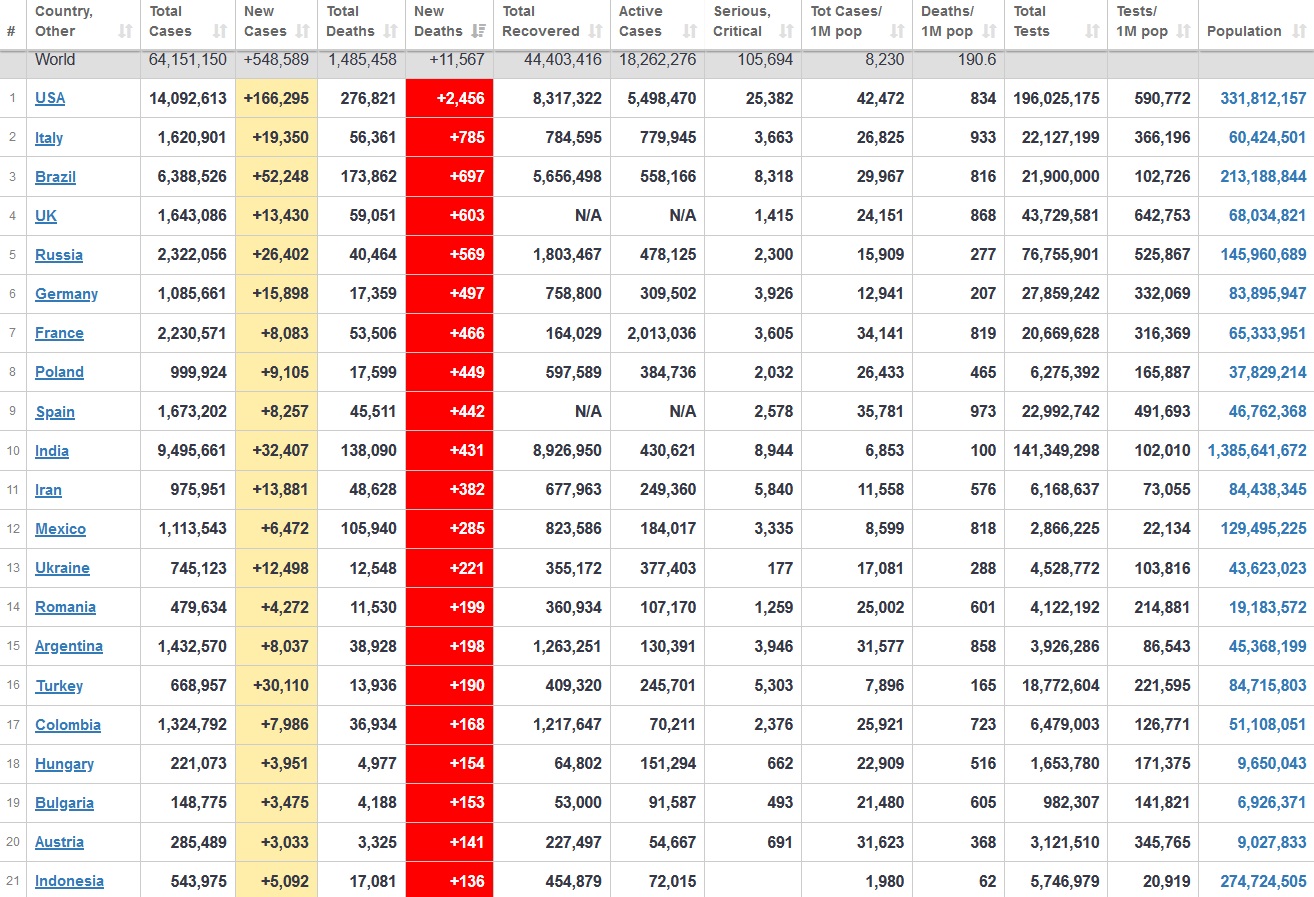
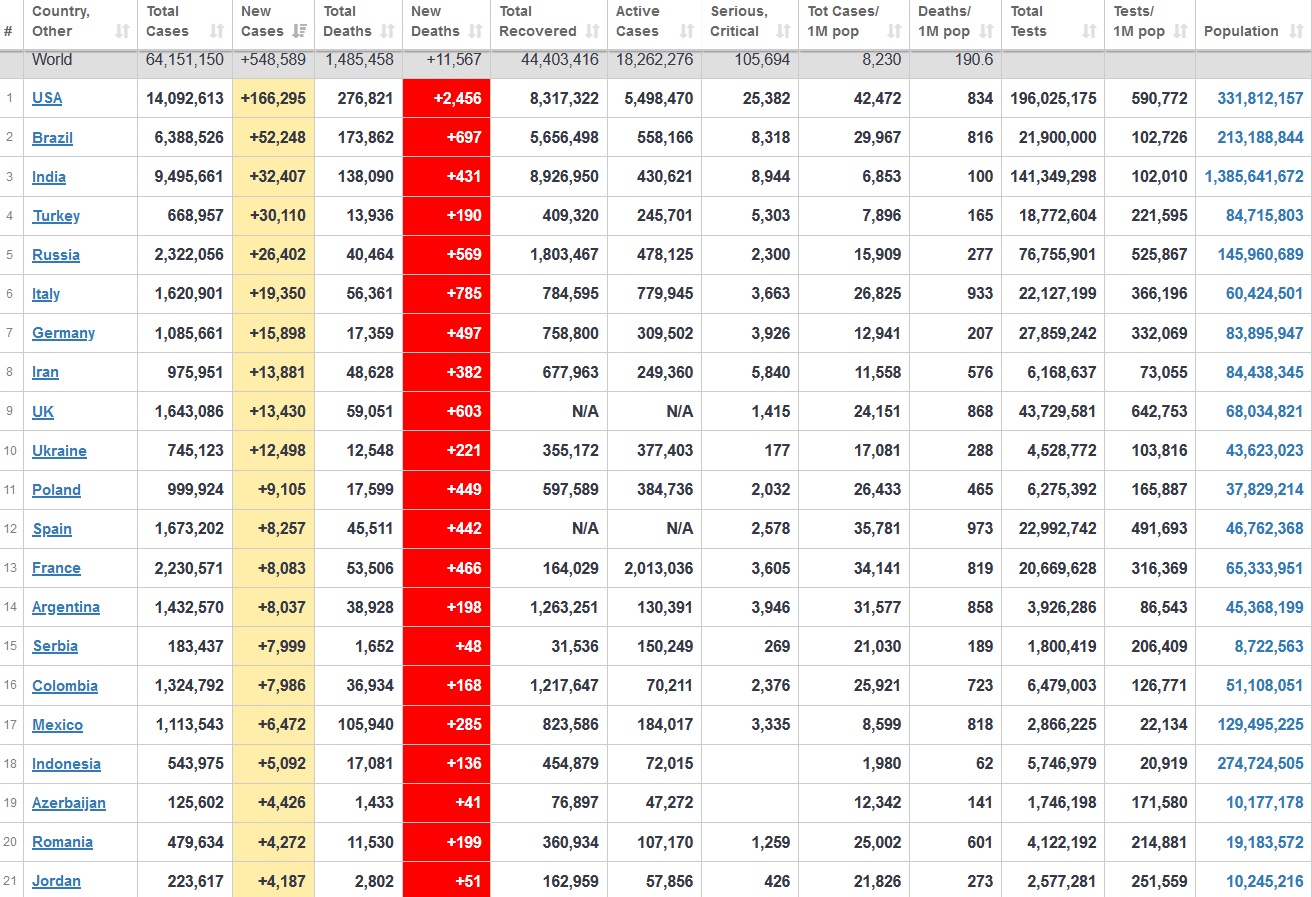
Does anyone know what computer systems the ONS etc use to collate their statistics please?
Rainbows
Jane
Rainbows
Jane
a reply to: angelchemuel
I think they get sent emails of the totals, and then collate all that data together.
I think they get sent emails of the totals, and then collate all that data together.
a reply to: MonkeyBalls2
Thank you. But I would love to know what kind of computer system/company they use to collate all those numbers on a daily basis......see wher I am going with this...wink, wink
Rainbows
Jane
Thank you. But I would love to know what kind of computer system/company they use to collate all those numbers on a daily basis......see wher I am going with this...wink, wink
Rainbows
Jane
RCT of Honey & Nigella Sativa Trial Against COVID-19 (HNS-COVID-PK) FINDINGS
HNS resulted in ~50% reduction in time taken to alleviate symptoms as compared to placebo (4 versus 7 days),
HNS also cleared the virus 4 days earlier than the placebo group in moderate (6 versus 10 days,]
and
In severe cases (8.5 days versus 12 days )
In severe cases, mortality rate was four-fold lower in HNS group than placebo (4% versus 18.87% )
Study on clinical trials site
Preprint on MedRxIV.Org
Benefits of Nigella Sativa
Look at the video from Dr Been
HNS resulted in ~50% reduction in time taken to alleviate symptoms as compared to placebo (4 versus 7 days),
HNS also cleared the virus 4 days earlier than the placebo group in moderate (6 versus 10 days,]
and
In severe cases (8.5 days versus 12 days )
In severe cases, mortality rate was four-fold lower in HNS group than placebo (4% versus 18.87% )
Study on clinical trials site
Preprint on MedRxIV.Org
Benefits of Nigella Sativa
Look at the video from Dr Been
Link to External peer review of the RTPCR test to detect SARS-CoV-2 reveals 10 major scientific flaws at
the molecular and methodological level: consequences for false positive results.
Basically, it puts into dispute, the efficacy of the PCR tests using this method (Amplifying the Data to get a result).
Abstract :
Basically, it puts into dispute, the efficacy of the PCR tests using this method (Amplifying the Data to get a result).
Abstract :
In the publication entitled “Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR” (Eurosurveillance 25(8) 2020) the authors present a diagnostic workflow and RT-qPCR protocol for detection and diagnostics of 2019-nCoV (now known as SARS-CoV-2), which they claim to be validated, as well as being a robust diagnostic methodology for use in public-health laboratory settings.
In light of all the consequences resulting from this very publication for societies worldwide, a group of independent researchers performed a point-by-point review of the aforesaid publication in which 1) all components of the presented test design were cross checked, 2) the RT-qPCR protocol-recommendations were assessed w.r.t. good laboratory practice, and 3) parameters examined against relevant scientific literature covering the field.
The published RT-qPCR protocol for detection and diagnostics of 2019-nCoV and the manuscript suffer from numerous technical and scientific errors, including insufficient primer design, a problematic and insufficient RT-qPCR protocol, and the absence of an accurate test validation. Neither the presented test nor the manuscript itself fulfils the requirements for an acceptable scientific publication. Further, serious conflicts of interest of the authors are not mentioned. Finally, the very short timescale between submission and acceptance of the publication (24 hours) signifies that a systematic peer review process was either not performed here, or of problematic poor quality. We provide compelling evidence of several scientific inadequacies, errors and flaws.
Considering the scientific and methodological blemishes presented here, we are confident that the editorial board of Eurosurveillance has no other choice but to retract the publication.
a reply to: MonkeyBalls2
That's the study JC discusses on his stream today at 16:30 mark --
says he is going to go into detail on Thursday.
That's the study JC discusses on his stream today at 16:30 mark --
says he is going to go into detail on Thursday.
a reply to: angelchemuel
Hi Jane, in the video the question of oil v powdered seed came up and I think the conclusion was the oil doesn't contain all the parts of the seed. Therefore may not work as well.
Suppose a bit like cod liver oil works best when consumed with a fat like proper milk
Hi Jane, in the video the question of oil v powdered seed came up and I think the conclusion was the oil doesn't contain all the parts of the seed. Therefore may not work as well.
Suppose a bit like cod liver oil works best when consumed with a fat like proper milk
So riddle me this....
We become the "FIRST COUNTRY IN THE WORLD" to roll out a vaccine for this 'Thing'........so where's China in the middle of all this?
They don't have a vaccine. Don't you think if this Thing was so bad CCP (China) would have been the first given they have a level 4 lab in WuHan where the outbreak happened? Don't you think the Chinese would have jumped all over this given they had a 'head start' and rolled one out months ago if not to make a 'killing' financially World wide?
Well lookee here, the Chinese are currently trialling their vaccine in 14 countries, yes human trials...
From the article below.
Please read the following article to understand the difference in the 4 types of vaccines currently being trialled world wide. Ours is a mRNA vaccine, completely 'novel' (just like the virus. Remember when it was called Novel Coronavirus?). This is completely new type of vaccine that has not been done before which will require 1-2 doses. Whereas the Chinese are working on the traditional 'whole, killed' vaccine' which required just the one dose, a tried and more trusted method of vaccination.
The attached article is from December 2nd.
edition.cnn.com...
vaccines
We become the "FIRST COUNTRY IN THE WORLD" to roll out a vaccine for this 'Thing'........so where's China in the middle of all this?
They don't have a vaccine. Don't you think if this Thing was so bad CCP (China) would have been the first given they have a level 4 lab in WuHan where the outbreak happened? Don't you think the Chinese would have jumped all over this given they had a 'head start' and rolled one out months ago if not to make a 'killing' financially World wide?
Well lookee here, the Chinese are currently trialling their vaccine in 14 countries, yes human trials...
"Because China has largely contained the virus, there's no urgent need to vaccinate every one of its 1.4 billion population. "That gives it this leverage ... to make deals with countries in need of the vaccines," he said."
From the article below.
Please read the following article to understand the difference in the 4 types of vaccines currently being trialled world wide. Ours is a mRNA vaccine, completely 'novel' (just like the virus. Remember when it was called Novel Coronavirus?). This is completely new type of vaccine that has not been done before which will require 1-2 doses. Whereas the Chinese are working on the traditional 'whole, killed' vaccine' which required just the one dose, a tried and more trusted method of vaccination.
The attached article is from December 2nd.
edition.cnn.com...
vaccines
new topics
-
Who's coming with me?
Predictions & Prophecies: 38 seconds ago -
FBI Director CHRISTOPHER WRAY Will Resign Before President Trump Takes Office on 1.20.2025.
US Political Madness: 3 hours ago -
The NIH is still sending taxpayer money to Chinese Labs to Conduct cruel animal experiments
Mainstream News: 3 hours ago -
Angela Merkel is terrified of dogs
Politicians & People: 5 hours ago -
Will all hell break out? Jersey drones - blue beam
Aliens and UFOs: 11 hours ago
top topics
-
The NIH is still sending taxpayer money to Chinese Labs to Conduct cruel animal experiments
Mainstream News: 3 hours ago, 11 flags -
FBI Director CHRISTOPHER WRAY Will Resign Before President Trump Takes Office on 1.20.2025.
US Political Madness: 3 hours ago, 7 flags -
Should be BANNED!
General Chit Chat: 13 hours ago, 6 flags -
Will all hell break out? Jersey drones - blue beam
Aliens and UFOs: 11 hours ago, 4 flags -
Angela Merkel is terrified of dogs
Politicians & People: 5 hours ago, 2 flags -
Who's coming with me?
Predictions & Prophecies: 38 seconds ago, 0 flags
active topics
-
Who's coming with me?
Predictions & Prophecies • 0 • : AlroyFarms -
Drones over New Jersey
Aliens and UFOs • 39 • : ratcals -
And Here Come the Excuses!!
General Conspiracies • 130 • : cherokeetroy -
Drones everywhere in New Jersey
Aliens and UFOs • 55 • : MetalThunder -
ILLUMINATION – Reverse Perception Of Cyclic Probability – CEO ASSASSINATION
Secret Societies • 20 • : KnowItAllKnowNothin -
Salvatore Pais confirms science in MH370 videos are real during live stream
General Conspiracies • 236 • : Lazy88 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 3611 • : 777Vader -
The NIH is still sending taxpayer money to Chinese Labs to Conduct cruel animal experiments
Mainstream News • 7 • : Thoughtful3 -
Old School Punk
Music • 562 • : underpass61 -
Will all hell break out? Jersey drones - blue beam
Aliens and UFOs • 33 • : CarlLaFong