It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Possibly. 2-4ml is the sample size for a lot of the flash point testers listed here but none of them look anything like that:
originally posted by: pteridine
originally posted by: EartOccupant
Here is a rough measurement in centimeters:
It would seem that the 5ml etch has to do with sample size.
Flash Point Testing explained, and various flash point testers (pdf)
originally posted by: Arbitrageur
Possibly. 2-4ml is the sample size for a lot of the flash point testers listed here but none of them look anything like that:
originally posted by: pteridine
originally posted by: EartOccupant
Here is a rough measurement in centimeters:
It would seem that the 5ml etch has to do with sample size.
Flash Point Testing explained, and various flash point testers (pdf)
The link is to a company that produces flash point test instruments so the designs are limited to their products. The glassware item in the OP may be stand alone, other than a thermostatic bath, or may be an insert into yet another instrument.
I used to buy up all sorts of lab equipment at auctions, you could get the oddest glassware. Half of it you'll never figure out. Made out like a
bandit on Ebay selling temperature baths, ultrasonic cleaners, distillation columns and the like. These days you can buy cheap Chinese ultrasonics so
there's no market there anymore, but the other lab stuff often goes for big bucks.
I'm going to guess it's something for distillation of a small sample. And I'm going to guess it's for things that can't be separated by the usual
method of heating. (Both substances evaporate and condense at almost the same temperatures. So the process needs to be done using a drop in
pressure.)
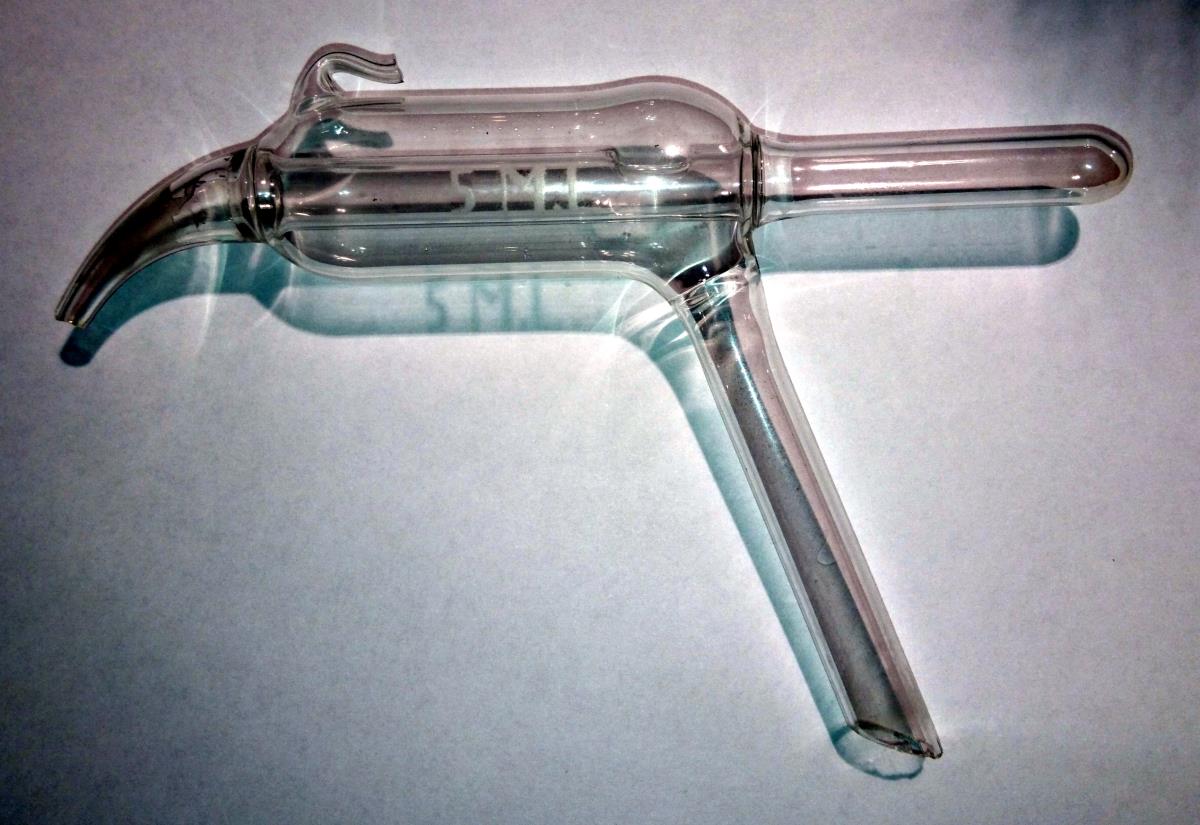
Looks like something that should have a cooling jacket on it. Cap the end of the spigot shaped part, and tilt up so the test-tube shaped part goes down. Tap or have a line going to the sample source with the angled part. Then hook a vacuum to the small hooked shaped part. When you hit a certain low enough pressure, the substance this thing tries to collect will start to evaporate out. By having cooling on this, eventually whatever you're getting a sample of will condense into the tube part. Then release the vacuum. Whatever you have collected in the tube end can now be poured out with the spigot end.
How that works into a whole process? Who knows. There are so many things done in chemistry.
Looks like something that should have a cooling jacket on it. Cap the end of the spigot shaped part, and tilt up so the test-tube shaped part goes down. Tap or have a line going to the sample source with the angled part. Then hook a vacuum to the small hooked shaped part. When you hit a certain low enough pressure, the substance this thing tries to collect will start to evaporate out. By having cooling on this, eventually whatever you're getting a sample of will condense into the tube part. Then release the vacuum. Whatever you have collected in the tube end can now be poured out with the spigot end.
How that works into a whole process? Who knows. There are so many things done in chemistry.
originally posted by: pauljs75
I'm going to guess it's something for distillation of a small sample. And I'm going to guess it's for things that can't be separated by the usual method of heating. (Both substances evaporate and condense at almost the same temperatures. So the process needs to be done using a drop in pressure.)
Looks like something that should have a cooling jacket on it. Cap the end of the spigot shaped part, and tilt up so the test-tube shaped part goes down. Tap or have a line going to the sample source with the angled part. Then hook a vacuum to the small hooked shaped part. When you hit a certain low enough pressure, the substance this thing tries to collect will start to evaporate out. By having cooling on this, eventually whatever you're getting a sample of will condense into the tube part. Then release the vacuum. Whatever you have collected in the tube end can now be poured out with the spigot end.
How that works into a whole process? Who knows. There are so many things done in chemistry.
We already know it's something used to determine flash point. To solve the problem of "both substances evaporate and condense at almost the same temperatures" one can use a fractional distillation column. Vacuum distillations are used for substances with higher boiling points at atmospheric pressure and fractional distillations under partial vacuum can be done, also.
It doesn't look like anything you would use for distillation. If it were a condenser for this purpose, it would be a terrible one. A typical reflux
condenser has a glass tube surrounded by another glass tube. These are sealed off from one another. The inner tube connects to the receiving flask and
the distillation vessel, where the outer tube would have cold water running through it to chill any vapour entering the inner tube, condensing it to
liquid and allowing it to flow into the receiving flask.
The tube in the picture has a hole in the inner tube. Not very useful for distillation. Moreover, the connections to the outer tube are not what you would typically see on a condenser.
It is nothing like any of the items in the picture.
I tried to look up what it might be, but the closest I got was something similar to glassware used for boiling point determination via Cottrell's method. There is no place for a thermometer, however. I cannot think how such an apparatus would be used for flash point. I might ask some colleagues to see if they know.
The tube in the picture has a hole in the inner tube. Not very useful for distillation. Moreover, the connections to the outer tube are not what you would typically see on a condenser.
originally posted by: blend57
a reply to: EartOccupant
Does it look similar to any of these?
To me it could be a distillation apparatus .. some sort of flash point apparatus..
The one marked "determining nitrogen in organic compounds or the cyanide distillation apparatus..
just thought I'd take a guess..
thanks,
blend57
It is nothing like any of the items in the picture.
I tried to look up what it might be, but the closest I got was something similar to glassware used for boiling point determination via Cottrell's method. There is no place for a thermometer, however. I cannot think how such an apparatus would be used for flash point. I might ask some colleagues to see if they know.
a reply to: hypervalentiodine
Tnx for your input!
I agree with it is not being a distillation kind of apparatus.
It don't makes sense when you follow the openings and stream lines of the gasses and or fluid.
Tnx for your input!
I agree with it is not being a distillation kind of apparatus.
It don't makes sense when you follow the openings and stream lines of the gasses and or fluid.
a reply to: EartOccupant
No problem. I often stumble across glassware in my lab whose sole purpose in life only seems to be to confuse the unsuspecting chemist. Some of them do prove to be useful, others just superfluous carry overs from times gone by.
No problem. I often stumble across glassware in my lab whose sole purpose in life only seems to be to confuse the unsuspecting chemist. Some of them do prove to be useful, others just superfluous carry overs from times gone by.
edit on 2-1-2017 by hypervalentiodine because: (no reason
given)
originally posted by: EartOccupant
I was on a second hand market the other day...
And i like lab stuff.. although i have not much knowledge of it.. : )
Anyway bought this:
The seller did say it was a "vlampunt bepaler" ( Dutch, something like: flame point determination )
I can not find anything about it, or the key words to google.
Anyone?
What is it, how to use it ?
It almost looks like an attachment for a Violet Wand (once used for shock therapy in the medical industry but now used for more deviant purposes) but have never seen one with a spout type appendage. I'd agree with others who are going along the lines of it being a distillation device. There are many different types and some have even been created specifically for one purpose, you may have a hard time finding out exactly what its purpose is...
hypervalentiodine
It's ok! That's why I said it was a guess.. I'm glad there is someone like you that is well versed in the subject that can help him out.. and I'm kind of curious as to what it could be now as well..so hopefully between you and others on here the mystery will be solved. I'll be following the thread...Can't wait to see what you guys finally come up with..thanks for the explanation!
Thanks,
blend57
It's ok! That's why I said it was a guess.. I'm glad there is someone like you that is well versed in the subject that can help him out.. and I'm kind of curious as to what it could be now as well..so hopefully between you and others on here the mystery will be solved. I'll be following the thread...Can't wait to see what you guys finally come up with..thanks for the explanation!
Thanks,
blend57
a reply to: EartOccupant
Wiki Wand Link for gas bubbler. I wanted to share this with you since most of my search for vlampunt bepalen led me to mostly metal apparatuses. I was wondering if your item might be more likely a gas bubbler? The picture at this link and description seems to fit more aptly.
Wiki Wand Link for gas bubbler. I wanted to share this with you since most of my search for vlampunt bepalen led me to mostly metal apparatuses. I was wondering if your item might be more likely a gas bubbler? The picture at this link and description seems to fit more aptly.
a reply to: CynConcepts
It wouldn't be a bubbler. The purpose of a gas bubbler is to provide a pressure release for some sort of gas, which doesn't allow back flow of atmospheric gas through the line. In a vey simple form, one might consist of an open glass joint that extends into a glass tube / vessel. The inner glass tube would go almost to the bottom of the vessel. The top of the vessel would have another outlet joint that goes to atmosphere. You fill the vessel with oil so that the excess gas bubbles through the oil and out of the outlet, but the atmospheric gasses cannot go back in.
The glassware shown here is not set up for that, and again, the glass joints are a bit weird.
It wouldn't be a bubbler. The purpose of a gas bubbler is to provide a pressure release for some sort of gas, which doesn't allow back flow of atmospheric gas through the line. In a vey simple form, one might consist of an open glass joint that extends into a glass tube / vessel. The inner glass tube would go almost to the bottom of the vessel. The top of the vessel would have another outlet joint that goes to atmosphere. You fill the vessel with oil so that the excess gas bubbles through the oil and out of the outlet, but the atmospheric gasses cannot go back in.
The glassware shown here is not set up for that, and again, the glass joints are a bit weird.
originally posted by: CynConcepts
a reply to: EartOccupant
Wiki Wand Link for gas bubbler. I wanted to share this with you since most of my search for vlampunt bepalen led me to mostly metal apparatuses. I was wondering if your item might be more likely a gas bubbler? The picture at this link and description seems to fit more aptly.
A gas bubbler is usually of much simpler construction. This is either a stand-alone flash point determination device or an insert for a flash point instrument.
new topics
-
Former Labour minister Frank Field dies aged 81
People: 12 minutes ago -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs: 1 hours ago -
This is our Story
General Entertainment: 4 hours ago -
President BIDEN Vows to Make Americans Pay More Federal Taxes in 2025 - Political Suicide.
2024 Elections: 6 hours ago -
Ode to Artemis
General Chit Chat: 7 hours ago -
Ditching physical money
History: 10 hours ago -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 11 hours ago -
Don't take advantage of people just because it seems easy it will backfire
Rant: 11 hours ago
top topics
-
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media: 15 hours ago, 14 flags -
President BIDEN Vows to Make Americans Pay More Federal Taxes in 2025 - Political Suicide.
2024 Elections: 6 hours ago, 10 flags -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 11 hours ago, 6 flags -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections: 12 hours ago, 6 flags -
Don't take advantage of people just because it seems easy it will backfire
Rant: 11 hours ago, 4 flags -
Ditching physical money
History: 10 hours ago, 4 flags -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs: 1 hours ago, 3 flags -
God lived as a Devil Dog.
Short Stories: 16 hours ago, 3 flags -
Ode to Artemis
General Chit Chat: 7 hours ago, 2 flags -
VirginOfGrand says hello
Introductions: 12 hours ago, 2 flags
active topics
-
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest • 266 • : KrustyKrab -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs • 12 • : Consvoli -
Former Labour minister Frank Field dies aged 81
People • 1 • : angelchemuel -
Russia Ukraine Update Thread - part 3
World War Three • 5720 • : Freeborn -
The Superstition of Full Moons Filling Hospitals Turns Out To Be True!
Medical Issues & Conspiracies • 23 • : confuzedcitizen -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media • 32 • : BedevereTheWise -
So this is what Hamas considers 'freedom fighting' ...
War On Terrorism • 227 • : HopeForTheFuture -
Remember These Attacks When President Trump 2.0 Retribution-Justice Commences.
2024 Elections • 51 • : Justoneman -
British TV Presenter Refuses To Use Guest's Preferred Pronouns
Education and Media • 131 • : FlyersFan -
Spectrophilia - Women Who Have Had Affairs With Ghosts Say Spooks Are Better Lovers Than Real Men
Paranormal Studies • 30 • : FlyersFan