It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Mary Rose
I'm wondering about whether or not there are members who've done their own evaluation of the laws of thermodynamics and have similar ideas of their own about what might need revision in view of present-day knowledge.
reply to post by Mary Rose
It can't be. If it could, there would be another definition for it. As zero point energy is the lowest energy state of a system. -Not sure how many times everyone has to say this to you.
The reason you even know the word "over unity" is because you frequent the free energy boards, discussions and watch the youtube videos, so it's all relevant, since over unity came about as marketing for all that crap.
And it simply is another term for perpetual motion. See above post: Either its a scientific term or it is a marketing term (which has no basis in science).
It's not a separate argument. What devices put out more than they cost to run? (energy cost, not consumer based monetary cost.)
*You are after all in the science forum.
Yes, I know there is that discussion, too. There is research going on about whether or not zero-point energy can be tapped.
It can't be. If it could, there would be another definition for it. As zero point energy is the lowest energy state of a system. -Not sure how many times everyone has to say this to you.
But it's not the discussion about overunity.
The reason you even know the word "over unity" is because you frequent the free energy boards, discussions and watch the youtube videos, so it's all relevant, since over unity came about as marketing for all that crap.
And it simply is another term for perpetual motion. See above post: Either its a scientific term or it is a marketing term (which has no basis in science).
It seems what we've established now is that the term "overunity" is not even in the dictionary. It redirects to "perpetual motion" and that's a separate argument altogether from devices that put out more than they cost to run.
It's not a separate argument. What devices put out more than they cost to run? (energy cost, not consumer based monetary cost.)
*You are after all in the science forum.
edit on 24-11-2013 by boncho because: (no reason given)
reply to post by Mary Rose
I do an evaluation of thermodynamics every time I get into my car.
If I post a macro that says, "Tesla invented sliced bread" it doesn't make the statement true. Where is the work that supposedly proves all these claims or is it simply a powerpoint presentation?
I'm wondering about whether or not there are members who've done their own evaluation of the laws of thermodynamics and have similar ideas of their own about what might need revision in view of present-day knowledge.
I do an evaluation of thermodynamics every time I get into my car.
If I post a macro that says, "Tesla invented sliced bread" it doesn't make the statement true. Where is the work that supposedly proves all these claims or is it simply a powerpoint presentation?
edit on 24-11-2013 by boncho because: (no reason given)
boncho
It can't be. If it could, there would be another definition for it. As zero point energy is the lowest energy state of a system. -Not sure how many times everyone has to say this to you.
If my memory serves, Haisch uses the term zero-point energy. You know who he is, right?
Mary Rose
It seems what we've established now is that the term "overunity" is not even in the dictionary. It redirects to "perpetual motion" and that's a separate argument altogether from devices that put out more than they cost to run.
And we've established that heat pumps in temperate climates are energy superstars.
The second law of thermodynamics has been violated on small scales but actually it's completely consistent with quantum theory.
Mary Rose
I'm wondering about whether or not there are members who've done their own evaluation of the laws of thermodynamics and have similar ideas of their own about what might need revision in view of present-day knowledge.
As someone said there are millions of scientists and engineers looking for holes in current theory, even though you appear to have been completely brainwashed by the pseudoscience charlatans to believe otherwise. And there's an example of where they found a hole in the second law, but it's a very tiny hole, literally, and it was predictable.
The analogy I'd use is if you walk up to a blackjack table, place a bet, and win, then walk away, you've violated the odds. The second law violation is kind of like that. If you keep playing blackjack, the house has the odds rigged in their favor, so over the long term you will lose, just as in thermodynamics, entropy will increase over the long run. But on a single hand of blackjack, or with a tiny thermodynamic system on a quantum scale, you can beat the odds for a short fleeting moment, sometimes.
Mary Rose
Mary Rose
It seems what we've established now is that the term "overunity" is not even in the dictionary. It redirects to "perpetual motion" and that's a separate argument altogether from devices that put out more than they cost to run.
And we've established that heat pumps in temperate climates are energy superstars.
On the consumer level not the scientific level. Stop mixing the terms.
Mary Rose
boncho
It can't be. If it could, there would be another definition for it. As zero point energy is the lowest energy state of a system. -Not sure how many times everyone has to say this to you.
If my memory serves, Haisch uses the term zero-point energy. You know who he is, right?
Yes but look at Haisch's work, he makes no claims on "sucking energy from ZPE" in fact he casts doubt on his own works and is working on the nano level with casimir.
reply to post by Arbitrageur
So, you have no suggestions for revisions, I take it.
Are you familiar at all with the work of Prigogine?
So, you have no suggestions for revisions, I take it.
Are you familiar at all with the work of Prigogine?
Mary Rose
reply to post by Arbitrageur
So, you have no suggestions for revisions, I take it.
Are you familiar at all with the work of Prigogine?
Are you familiar with the work of Lars Onsager?
I have a feeling Mary is about to tell us the proof and method for immortality is Schrodingers Cat.
Mary Rose
Are you familiar at all with the work of Prigogine?
Mary Rose
And do you agree that there is equilibrium and non-equilibrium thermodynamics?
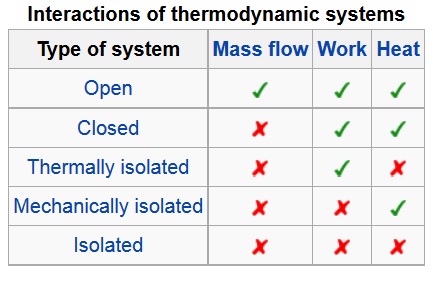
Here’s the screenshot I previously posted but has not yet been discussed:

The Free Dictionary redirects to “dissipative system” and The American Heritage Science Dictionary definition of “open system” if you type “dissipative structures.” If you click on the link for "open system" you get this:
open system
A physical system that interacts with other systems. The physical description of an open system can appear to violate conservation laws; for example, in a good description of the mechanism of energy transfer in a car engine (gears, driveshaft, and so on), energy will appear to be lost from the system over time, despite the law of conservation of energy. This is because the system is open, losing energy (in the form of heat) to surrounding systems (through friction). A system that loses energy in this way also called a dissipative system. Compare closed system.
The American Heritage® Science Dictionary Copyright © 2005 by Houghton Mifflin Company. Published by Houghton Mifflin Company. All rights reserved.
It says to also see their Medical, Encyclopedia, and Wikipedia dictionaries.
Continuing, if you search Wikipedia for "open system," and read what it says for the thermodynamic meaning of the term, it says the following, and links to the article "Thermodynamic system," which it says needs additional citations.
Open system (thermodynamics), in thermodynamics and physics, a system where matter and energy can enter or leave, in contrast to a closed system where energy can enter or leave but matter can not
It seems to me that well-established science for something as important as "thermodynamic system" would not have an article that needs citations.
I previously posted:
Mary Rose
The Wikipedia article "Thermodynamic system" says it needs additional citations for verification, but assuming for the time being that the article is accurate: For the particular scenario the NIST article is referring to, which thermodynamic system do the heat pumps in question fit in to?
Open, right?
I posted it because of the article by the National Institute of Standards and Technology (NIST) about the "typical " heat pump as an energy superstar.
Arbitrageur has posted this on the topic:
Arbitrageur
I would use the terms along the same lines as the authors of this paper:
Open and closed-cycle mechanical vapour-compression heat-pump assisted sea-water purification systems
Using the sloppy definitions in the wiki, one might misconstrue both the open and closed system as open systems, but I think the authors of the paper use the terms correctly (or at least the way I would) to differentiate between open and closed systems.
Note NIST was just testing the heat pump, not a whole thermodynamic system, and the same heat pump could be used in either an open or closed system.
What I would like to do now is focus on the big picture of the laws of thermodynamics and what possibly needs modification, not just heat pumps as a case-in-point.
But perhaps that's another thread.
edit on 11/24/13 by Mary Rose because: Add link
reply to post by Mary Rose
Did you read the article?
Heat pumps don't need modification. Your understanding of them does.
Did you read the article?
It's a press release to the public speaking in laymen terms, it's different than scientific language. We have been trying to drill this into your head for awhile now.
Media Contact: John Blair, [email protected], xxx-xxx-xxxx
What I would like to do now is focus on the big picture of the laws of thermodynamics and what possibly needs modification, not just heat pumps as a case-in-point.
But perhaps that's another thread.
Heat pumps don't need modification. Your understanding of them does.
edit on 24-11-2013 by boncho because: (no reason given)
reply to post by Mary Rose
Heat pumps are a thermodynamic cycle, or thermodynamic process. To calculate efficiency for this it is expressed
er = 1 –. T. T. H. C.
For clarification, a set of processes or a *cycle* can make up a larger interpretation of a thermodynamic system.
dev5.mhhe.com...
Now, if you add work out, it is much easier to describe. Which is why coefficient of performance is used.
Or a hypothetical reverse heat pump is considered.
The Wikipedia article "Thermodynamic system" says it needs additional citations for verification, but assuming for the time being that the article is accurate: For the particular scenario the NIST article is referring to, which thermodynamic system do the heat pumps in question fit in to?
Heat pumps are a thermodynamic cycle, or thermodynamic process. To calculate efficiency for this it is expressed
er = 1 –. T. T. H. C.
For clarification, a set of processes or a *cycle* can make up a larger interpretation of a thermodynamic system.
dev5.mhhe.com...
Now, if you add work out, it is much easier to describe. Which is why coefficient of performance is used.
Or a hypothetical reverse heat pump is considered.
edit on 24-11-2013 by boncho because: (no reason given)
reply to post by Mary Rose
It's a wiki page. You know how many times Bearden fans have come in and completely shredded articles claiming that free energy exists? Besides, citations are citations, something in there can be true or false with or without citations.
If you want, simply stop using wiki and use other sources.
It seems to me that well-established science for something as important as "thermodynamic system" would not have an article that needs citations.
It's a wiki page. You know how many times Bearden fans have come in and completely shredded articles claiming that free energy exists? Besides, citations are citations, something in there can be true or false with or without citations.
If you want, simply stop using wiki and use other sources.
Mary Rose
Isn't the increased efficiency due to the recovery of wasted energy a way to get back more than you put in without spending more money with increased electricity in?
No because it is still a part of what you put in.
In other words, the electricity/energy put in, does remain the same and that's the whole point. That's what you're trying to accomplish.
It still isn't overunity.
reply to post by Mary Rose
To help you with the differences in thermodynamic systems and processes, or cycle (which can go through multiple processes but arrive back at the original one)… this should help:
Source
And it's not wiki.
No citation problems.
Also, for a very basic understanding of open, closed and isolated systems.
Link
To help you with the differences in thermodynamic systems and processes, or cycle (which can go through multiple processes but arrive back at the original one)… this should help:
The first and an extremely important step in the study of thermodynamics is to choose and identify the system properly and show the system boundaries clearly.
A process is defined as the path of thermodynamic states which the system passes through as it goes from an initial state to a final state. In refrigeration and air conditioning one encounters a wide variety of processes. Understanding the nature of the process path is very important as heat and work depend on the path.
A system is said to have undergone a cycle if beginning with an initial state it goes through different processes and finally arrives at the initial state.
Source
And it's not wiki.
No citation problems.
Also, for a very basic understanding of open, closed and isolated systems.
Link
Thermodynamic systems:
Thermodynamic systems can be either closed, or open or isolated.
Closed systems: A closed system is defined when a particular quantity of matter is under study. A closed system always contains the same matter. There can be no mass transfers across the boundary. There may be energy transfer across the boundary.
Open systems. A closed system is defined when a fixed volume is under study. There can be mass transfers as well as energy transfers across the boundary.
Isolated systems: An isolated system is special type of closed system that does not even transfer energy across the boundary. There will be no interactions with the surroundings.
For example, the contents of a pressure cooker on a stove with its lid tightly closed and the whistle in position, is a closed system as no mass can enter or leave the pressure cooker, but heat can be transferred to it. When the whistle of the pressure cooker blows, then it becomes an open system as steam leaves the cooker. A perfectly insulated, rigid and closed vessel is an example of an isolated system as neither mass nor energy can enter or leave the system.
daskakik
Mary Rose
Isn't the increased efficiency due to the recovery of wasted energy a way to get back more than you put in without spending more money with increased electricity in?
No because it is still a part of what you put in.
In other words, the electricity/energy put in, does remain the same and that's the whole point. That's what you're trying to accomplish.
It still isn't overunity.
Be careful with her here. Anytime she mentions money, the discussion becomes something entirely different.
Mary will say,
$5 + 60kWh vs. $10 + 45kWh = Number 1 is over unity.
Money is not used in scientific calculations.
edit on 24-11-2013 by boncho because: (no reason given)
boncho
It can't be.
Yes, it can.
It's still energy present when it theoretically shouldn't be there at the temperature absolute zero.
boncho
It's a press release to the public speaking in laymen terms, it's different than scientific language.
Irrelevant.
The layman wants to know if the heat pump is not a superstar in their climate.
boncho
Heat pumps don't need modification. Your understanding of them does.
That’s not what I said.
Thermodynamics needs modification.
reply to post by boncho
My question was referring to Open, Closed, Thermally isolated, Mechanically isolated, or Isolated, as listed in the chart in the Wikipedia article.
reply to post by boncho
Wikipedia is a poor source for anything that is controversial.
It is very mainstream. One would think that if thermodynamics were well-understood and well-established that there would be a good article on it, as there usually is, if the subject is not controversial.
reply to post by boncho
Thank you very much. I haven't read the .pdf yet but it does look to be a good source.
boncho
Money is not used in scientific calculations.
Of course not.
Closed systems: A closed system is defined when a particular quantity of matter is under study. A closed system always contains the same matter. There can be no mass transfers across the boundary.
I suspect that that system is probably not well-understood, because I don't think that mass is well-understood.
new topics
-
Electrical tricks for saving money
Education and Media: 3 hours ago -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 4 hours ago -
Sunak spinning the sickness figures
Other Current Events: 5 hours ago -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues: 5 hours ago -
Late Night with the Devil - a really good unusual modern horror film.
Movies: 7 hours ago -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 8 hours ago -
The Good News According to Jesus - Episode 1
Religion, Faith, And Theology: 10 hours ago
top topics
-
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 8 hours ago, 8 flags -
Florida man's trip overseas ends in shock over $143,000 T-Mobile phone bill
Social Issues and Civil Unrest: 15 hours ago, 8 flags -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 4 hours ago, 8 flags -
Former Labour minister Frank Field dies aged 81
People: 17 hours ago, 4 flags -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three: 12 hours ago, 3 flags -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues: 5 hours ago, 3 flags -
Sunak spinning the sickness figures
Other Current Events: 5 hours ago, 3 flags -
Bobiverse
Fantasy & Science Fiction: 15 hours ago, 3 flags -
Electrical tricks for saving money
Education and Media: 3 hours ago, 3 flags -
Late Night with the Devil - a really good unusual modern horror film.
Movies: 7 hours ago, 2 flags
active topics
-
Electrical tricks for saving money
Education and Media • 3 • : Mike72 -
TLDR post about ATS and why I love it and hope we all stay together somewhere
General Chit Chat • 10 • : theshadowknows -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News • 31 • : WeMustCare -
Why to avoid TikTok
Education and Media • 20 • : 5thHead -
How ageing is" immune deficiency"
Medical Issues & Conspiracies • 32 • : annonentity -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 657 • : daskakik -
President BIDEN Vows to Make Americans Pay More Federal Taxes in 2025 - Political Suicide.
2024 Elections • 135 • : WeMustCare -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 30 • : DaRAGE -
Spectrophilia - Women Who Have Had Affairs With Ghosts Say Spooks Are Better Lovers Than Real Men
Paranormal Studies • 32 • : burritocat -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest • 18 • : Xtrozero