It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
reply to post by TheRedneck
When incoming sunlight goes through the atmosphere the infrared frequencies which you mentioned are absorbed by CO2. Also, the shorter wavelengths which make it through the atmosphere hit the Earth's surface and heat it. That heat is re-radiated back (at longer wavelengths) toward the sky where those correct wavelengths are also absorbed by CO2. What wavelengths does an asphalt parking lot radiate? The sands of a desert heated by the Sun all day long?
The more CO2 (and other GHGs) there is in the atmosphere, the more heating of the atmosphere by both incoming and outgoing infrared.
What is your source of information for this?
The 4.3 micrometer band is what is responsible for the runaway global warming on Venus... it radiates a lot of energy at that wavelength which gets absorbed and re-emitted back to the planet.
That is within the band we established earlier, corresponding to the coldest temperatures ever recorded, but a long way from the temperatures we consider 'normal'.
When incoming sunlight goes through the atmosphere the infrared frequencies which you mentioned are absorbed by CO2. Also, the shorter wavelengths which make it through the atmosphere hit the Earth's surface and heat it. That heat is re-radiated back (at longer wavelengths) toward the sky where those correct wavelengths are also absorbed by CO2. What wavelengths does an asphalt parking lot radiate? The sands of a desert heated by the Sun all day long?
The more CO2 (and other GHGs) there is in the atmosphere, the more heating of the atmosphere by both incoming and outgoing infrared.
edit on 6/24/2013 by Phage because: (no reason given)
reply to post by Phage
The atmosphere of Venus is composed primarily of CO2 and nitrogen. The surface temperature is estimated at over 450 C, which equates to a maximum wavelength frequency of 4.0 micrometers. The 4.3 micrometer absorption band of CO2 is close to this value; hence if the CO2 greenhouse effect is responsible for the high surface temperatures, which seems likely to me, it would be because of the 4.3 micrometer absorption band.
That depends on their temperature. You know that.
If I spit in the ocean, I raise the sea level.
TheRedneck
What is your source of information for this?
The atmosphere of Venus is composed primarily of CO2 and nitrogen. The surface temperature is estimated at over 450 C, which equates to a maximum wavelength frequency of 4.0 micrometers. The 4.3 micrometer absorption band of CO2 is close to this value; hence if the CO2 greenhouse effect is responsible for the high surface temperatures, which seems likely to me, it would be because of the 4.3 micrometer absorption band.
What wavelengths does an asphalt parking lot radiate? The sands of a desert heated by the Sun all day long?
That depends on their temperature. You know that.
The more CO2 (and other GHGs) there is in the atmosphere, the more heating of the atmosphere by both incoming and outgoing infrared.
If I spit in the ocean, I raise the sea level.
TheRedneck
I have no doubt that the measurements of infrared radiation are increasing; I accept those findings at this time. I do have an issue with it being
attributed solely to CO2. Water vapor has an extremely wide absorption spectrum that covers almost the entire range of planetary radiation and CH4 is
very well aligned with this radiation spectrum.
Saying CO2 is more than minimally responsible for global warming is no different than a salesman selling you a clear window pane for a sunshade.
TheRedneck
Saying CO2 is more than minimally responsible for global warming is no different than a salesman selling you a clear window pane for a sunshade.
TheRedneck
reply to post by poet1b
and where would this safe place be located? just curious as to where
others think a good spot would be.
and where would this safe place be located? just curious as to where
others think a good spot would be.
reply to post by TheRedneck
You seem to have a bit of misunderstanding of what blackbody radiation is. The blackbody "temperature" is that wavelength at which the emission of radiation is maximized. It doesn't mean that it is the only wavelength which is emitted. Looking at the image below you can see that a temperature of 450ºC (723ºK) has a peak emissivity at about 4µm but it also is emitting at a wide range of other wavelengths, as do lower temperatures. Wavelengths which will be absorbed by CO2.
hence if the CO2 greenhouse effect is responsible for the high surface temperatures, which seems likely to me, it would be because of the 4.3 micrometer absorption band.
Yes. They get pretty hot don't they? Where do you think that heat goes? The more CO2 there is the more of it is captured by the atmosphere instead of radiating back into space. No one is saying that Earth will get as hot as Venus. But it doesn't have to for the climate to change dramatically.
That depends on their temperature. You know that.
26 gigatons (a year of CO2) is one hell of a loogie.
If I spit in the ocean, I raise the sea level.
edit on 6/24/2013 by Phage because: (no reason given)
reply to post by Phage
No, I fully understand that the frequencies vary widely from that peak frequency. That's why, despite the peak frequency of the earth being at 11.4 um, I admit that CO2 does absorb and re-radiate some heat at 15 um. I simply state that it ONLY absorbs the portion of radiation at 15 um, which is a tiny percentage of the entire radiation spectrum.
If you want to say that it can absorb on the 4.3 um band due to the earth's bandwidth, I would have to say that is true as well theoretically. But the amount of radiation from the earth coincidental with that absorption spectrum is so microscopic as to be completely negligible in the extreme.
The heat is radiated back into space. If a 15 um wavelength of that heat meets up with a molecule of CO2 (or water vapor) it will be absorbed and re-emitted in a random direction. That's a 50% probability that it will return to earth (assuming a low enough altitude for the planet to be considered a plane... just to take care of any nit-pickers) and add to the heat received.
Incidentally, the hotter the surface, the farther the peak wavelength is from the CO2 absorption band, and thus the smaller the percentage of energy in that band.
A tiny percentage (I have heard the figure of 0.8%, but I am not sure of that) of the energy has the opportunity to intercept 0.04% of the atmosphere it travels through. That's not exactly a heat lamp hiding in the atmosphere. It's more like 3 watts per square meter average if every bit of that 0.8% is reflected by 0.04% of the atmosphere.
People have used Venus as a scare tactic to get public acceptance of global warming (I think Al Gore used it in An Inconvenient Truth). That is one reason why I used the analogy in the explanation.
The atmosphere is one hell of a big ocean (5 million gigatons?).
26 gigatons is 0.00052% of that. Wow... perspective...
TheRedneck
You seem to have a bit of misunderstanding of what blackbody radiation is.
No, I fully understand that the frequencies vary widely from that peak frequency. That's why, despite the peak frequency of the earth being at 11.4 um, I admit that CO2 does absorb and re-radiate some heat at 15 um. I simply state that it ONLY absorbs the portion of radiation at 15 um, which is a tiny percentage of the entire radiation spectrum.
If you want to say that it can absorb on the 4.3 um band due to the earth's bandwidth, I would have to say that is true as well theoretically. But the amount of radiation from the earth coincidental with that absorption spectrum is so microscopic as to be completely negligible in the extreme.
Where do you think that heat goes? The more CO2 there is the more of it is captured by the atmosphere instead of radiating back into space. No one is saying that Earth will get as hot as Venus. But it doesn't have to for the climate to change dramatically.
The heat is radiated back into space. If a 15 um wavelength of that heat meets up with a molecule of CO2 (or water vapor) it will be absorbed and re-emitted in a random direction. That's a 50% probability that it will return to earth (assuming a low enough altitude for the planet to be considered a plane... just to take care of any nit-pickers) and add to the heat received.
Incidentally, the hotter the surface, the farther the peak wavelength is from the CO2 absorption band, and thus the smaller the percentage of energy in that band.
A tiny percentage (I have heard the figure of 0.8%, but I am not sure of that) of the energy has the opportunity to intercept 0.04% of the atmosphere it travels through. That's not exactly a heat lamp hiding in the atmosphere. It's more like 3 watts per square meter average if every bit of that 0.8% is reflected by 0.04% of the atmosphere.
People have used Venus as a scare tactic to get public acceptance of global warming (I think Al Gore used it in An Inconvenient Truth). That is one reason why I used the analogy in the explanation.
26 gigatons (a year of CO2) is one hell of a loogie.
The atmosphere is one hell of a big ocean (5 million gigatons?).
26 gigatons is 0.00052% of that. Wow... perspective...
TheRedneck
reply to post by TheRedneck
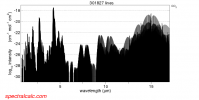
Compare that absorption spectrum to the curve for 300ºK. You can interpolate to 350º if you like but I don't think it will get that hot any time soon. That would really take a lot of CO2.
Then you are simply wrong. There are only a couple of (infrared) wavelengths at which CO2 does not absorb radiation.
I simply state that it ONLY absorbs the portion of radiation at 15 um, which is a tiny percentage of the entire radiation spectrum.

Compare that absorption spectrum to the curve for 300ºK. You can interpolate to 350º if you like but I don't think it will get that hot any time soon. That would really take a lot of CO2.
What about the next CO2 molecule it encounters? That's why more CO2=more heat. The heat is retained because the radiation bounces around between GHGs. There is also the fact that a "hot" CO2 molecule which comes into contact with any other molecule will transfer heat in the form of kinetic energy.
The heat is radiated back into space. If a 15 um wavelength of that heat meets up with a molecule of CO2 (or water vapor) it will be absorbed and re-emitted in a random direction. That's a 50% probability that it will return to earth (assuming a low enough altitude for the planet to be considered a plane... just to take care of any nit-pickers) and add to the heat received.
Yes. But there is still energy there. Energy which CO2 is all too happy to absorb. And it will keep absorbing it until it peaks and emmits (when it will begin absorbing again) or bumps into another molecule of, say nitrogen or oxygen, and heats it up.
Incidentally, the hotter the surface, the farther the peak wavelength is from the CO2 absorption band, and thus the smaller the percentage of energy in that band.
People have used Venus as an example of how atmospheric absorbtion works. Unless you are saying that people have said "If we don't do something Earth will be like Venus!"
People have used Venus as a scare tactic to get public acceptance of global warming (I think Al Gore used it in An Inconvenient Truth).
And the percentage of CO2 is rising, steadily. Are you really trying to make the point that it doesn't matter how much CO2 is in the atmosphere? That it won't cause a temperature increase? That CO2 cannot affect radiative forcing?
26 gigatons is 0.00052% of that. Wow... perspective...
edit on 6/24/2013 by Phage because: (no reason given)
Yes this is the big cycle, I'd say. Methane is the real worry for if it all suddenly is released its possibly going to be a big die off on earth.
reply to post by Phage
Me and a whole bunch of physics textbooks, apparently.
Your image has nothing to do with CO2 absorption., It is the radiation curve for different blackbody temperatures.
It is re-emitted again. It's still a single photon of IR energy, regardless of how many times it encounters a CO2 molecule, until it strikes the planet surface (or another object that does not re-emit it).
Please tell me you don't think CO2 is magically creating heat energy...
As far apart as molecules are in the atmosphere, it is unlikely that kinetic energy is going to transfer in the microsecond or so between absorption and re-emission.
As far as re-absorbing... only one photon is absorbed at a time. When it is re-emitted, another can be absorbed. In order to absorb more than one photon, there has to be another energy level... and there is, but it is pretty high in CO2 and not normally attained.
The emission is also of the same wavelength as the absorption. CO2 is not an energy sponge; it simply can absorb and re-emit certain wavelengths one at a time.
That was the insinuation.
How long have i got to spit in the ocean to flood New York City?
I assume you will agree with me on one point here: the ecosystem is extremely important to reabsorbing CO2 both to maintain a reasonable atmospheric level and to produce sufficient oxygen from that CO2. I support maintaining the rain forests both for their CO2 sink properties and to preserve the variety of flora and fauna indigenous to them. The oceans, however, specifically plankton, are the greatest CO2 sink and oxygen supplier of all. So far I see nothing that is endangering oceanic flora.
If we lose all natural CO2 sinks and continue pumping it into the air, then we will be in serious trouble. Not from the temperatures, but simply because we will all asphyxiate before we can realize it's getting hot. Luckily, that is about as reasonable a scenario as Al Gore having an intelligent thought process.
TheRedneck
Then you are simply wrong.
Me and a whole bunch of physics textbooks, apparently.
Your image has nothing to do with CO2 absorption., It is the radiation curve for different blackbody temperatures.
What about the next CO2 molecule it encounters?
It is re-emitted again. It's still a single photon of IR energy, regardless of how many times it encounters a CO2 molecule, until it strikes the planet surface (or another object that does not re-emit it).
Please tell me you don't think CO2 is magically creating heat energy...
Yes. But there is still energy there. Energy which CO2 is all too happy to absorb. And it will keep absorbing it until it peaks and emmits (when it will begin absorbing again) or bumps into another molecule of, say nitrogen or oxygen, and heats it up.
As far apart as molecules are in the atmosphere, it is unlikely that kinetic energy is going to transfer in the microsecond or so between absorption and re-emission.
As far as re-absorbing... only one photon is absorbed at a time. When it is re-emitted, another can be absorbed. In order to absorb more than one photon, there has to be another energy level... and there is, but it is pretty high in CO2 and not normally attained.
The emission is also of the same wavelength as the absorption. CO2 is not an energy sponge; it simply can absorb and re-emit certain wavelengths one at a time.
People have used Venus as an example of how atmospheric absorbtion works. Unless you are saying that people have said "If we don't do something Earth will be like Venus!"
That was the insinuation.
And the percentage of CO2 is rising, steadily.
How long have i got to spit in the ocean to flood New York City?
I assume you will agree with me on one point here: the ecosystem is extremely important to reabsorbing CO2 both to maintain a reasonable atmospheric level and to produce sufficient oxygen from that CO2. I support maintaining the rain forests both for their CO2 sink properties and to preserve the variety of flora and fauna indigenous to them. The oceans, however, specifically plankton, are the greatest CO2 sink and oxygen supplier of all. So far I see nothing that is endangering oceanic flora.
If we lose all natural CO2 sinks and continue pumping it into the air, then we will be in serious trouble. Not from the temperatures, but simply because we will all asphyxiate before we can realize it's getting hot. Luckily, that is about as reasonable a scenario as Al Gore having an intelligent thought process.
TheRedneck
reply to post by TheRedneck
spectralcalc.com...
No. It is the absorption spectrum for CO2.
Your image has nothing to do with CO2 absorption., It is the radiation curve for different blackbody temperatures.
spectralcalc.com...
Really? Then why does the air get warmer when the Sun is out? What heats up all that nitrogen and oxygen?
As far apart as molecules are in the atmosphere, it is unlikely that kinetic energy is going to transfer in the microsecond or so between absorption and re-emission.
We aren't talking about a flood.
How long have i got to spit in the ocean to flood New York City?
If CO2 levels get high enough to kill us the world will be a very hot place.
If we lose all natural CO2 sinks and continue pumping it into the air, then we will be in serious trouble. Not from the temperatures, but simply because we will all asphyxiate before we can realize it's getting hot.
Originally posted by TheRedneck
I assume you will agree with me on one point here: the ecosystem is extremely important to reabsorbing CO2 both to maintain a reasonable atmospheric level and to produce sufficient oxygen from that CO2. I support maintaining the rain forests both for their CO2 sink properties and to preserve the variety of flora and fauna indigenous to them. The oceans, however, specifically plankton, are the greatest CO2 sink and oxygen supplier of all. So far I see nothing that is endangering oceanic flora.
TheRedneck
The oceans getting more acid because of the high current levels of co2. At 1 point the ocean will not absorb co2 but will even add more co2 to the atmosphere. So now you already see for some time, corals getting wiped out because of the higher ph (more acid) (see first link below).
Right now, jellyfish are on the rise, since things are out of balance.
We had that 1 time before, a loooooong time ago where jellyfish ruled the oceans, we may head in the direction (again) (see second link).
www.guardian.co.uk...
www.cbc.ca...
edit on 24-6-2013 by Plugin because: (no reason
given)
reply to post by Phage
Whoops, was looking at the wrong image... try setting the resolution to linear instead of logarithmic:
You're talking a completely different process. Conduction and convection instead of radiation.
It's called an analogy.
TheRedneck
No. It is the absorption spectrum for CO2.
Whoops, was looking at the wrong image... try setting the resolution to linear instead of logarithmic:
Really? Then why does the air get warmer when the Sun is out? What heats up all that nitrogen and oxygen?
You're talking a completely different process. Conduction and convection instead of radiation.
We aren't talking about a flood.
It's called an analogy.
TheRedneck
reply to post by Plugin
Look at sulfur levels.
Excuse me while I drink some carbonic acid... I mean Mountain Dew....
TheRedneck
Look at sulfur levels.
Excuse me while I drink some carbonic acid... I mean Mountain Dew....
TheRedneck
reply to post by TheRedneck
Blackbody radiation for 300ºK extends across that range. CO2 absorbs energy across that range (except for two very narrow bands).
Yes. I know. There are three major peaks with absorbtion across the spectral range. When the linear scale is used the lower absorbion amounts disappear but they are still there. If the upper limit could be adjusted you would see them. You said there is only absorbtion at 15µm.
Whoops, was looking at the wrong image... try setting the resolution to linear instead of logarithmic:
Blackbody radiation for 300ºK extends across that range. CO2 absorbs energy across that range (except for two very narrow bands).
What is conduction if not a transfer of kinetic energy from one molecule to the next? Or are you saying that only air molecules which contact the ground get heated?
You're talking a completely different process. Conduction and convection instead of radiation.
Sort of like Venus then?
It's called an analogy.
edit on 6/25/2013 by Phage because: (no reason given)
reply to post by TheRedneck
Sorry for responding so late and thanks for the link to the studies being done on the volcanic activity in the arctic however I asked if there were any scientists or peer reviewed papers that support your theory that the arctic melt is being caused by those volcanos.
Was your reply meant to imply there are no such articles or scientists that support your theory?
Sorry for responding so late and thanks for the link to the studies being done on the volcanic activity in the arctic however I asked if there were any scientists or peer reviewed papers that support your theory that the arctic melt is being caused by those volcanos.
Was your reply meant to imply there are no such articles or scientists that support your theory?
reply to post by Phage
The reason the absorption at other wavelengths doesn't show up on a linear scaling is that it s minuscule and irrelevant. Every day on earth, there are hundreds of earthquakes so small they are never felt, and probably thousands so small they don't even register. Should we panic every day because we are having so many earthquakes?
There is such a thing as relevancy. You, of all people, should know this. I expected much better from you, Phage.
A method to try and discredit facts shown... apparently. See above.
Yes.
TheRedneck
You said there is only absorbtion at 15µm.
The reason the absorption at other wavelengths doesn't show up on a linear scaling is that it s minuscule and irrelevant. Every day on earth, there are hundreds of earthquakes so small they are never felt, and probably thousands so small they don't even register. Should we panic every day because we are having so many earthquakes?
There is such a thing as relevancy. You, of all people, should know this. I expected much better from you, Phage.
What is conduction
A method to try and discredit facts shown... apparently. See above.
Sort of like Venus then?
Yes.
TheRedneck
reply to post by Grimpachi
So far as I know, no one has yet been able to make a detailed mapping of the seafloor under the ice sheet. No climatologists have done any serious research into the area, and geologists are still trying to understand how the activity can occur at such depths and pressures. Until the discovery of volcanic activity, it was believed volcanic venting at those depths was impossible.
So no, no one has done a detailed investigation into how much heat is being produced as of yet. Hopefully more data will be forthcoming soon.
TheRedneck
So far as I know, no one has yet been able to make a detailed mapping of the seafloor under the ice sheet. No climatologists have done any serious research into the area, and geologists are still trying to understand how the activity can occur at such depths and pressures. Until the discovery of volcanic activity, it was believed volcanic venting at those depths was impossible.
So no, no one has done a detailed investigation into how much heat is being produced as of yet. Hopefully more data will be forthcoming soon.
TheRedneck
reply to post by TheRedneck
Just wanted to express to you my support of your conclusion that CO2 cannot be responsible alone for warming, as shown in the post-1940s temperature drop while CO2 level was rising.
Just wanted to express to you my support of your conclusion that CO2 cannot be responsible alone for warming, as shown in the post-1940s temperature drop while CO2 level was rising.
reply to post by TheRedneck
Slight yes, irrelevant no. They represent absorption of energy.
The reason the absorption at other wavelengths doesn't show up on a linear scaling is that it s minuscule and irrelevant.
Now that is irrelevant. We aren't talking about earthquakes we are talking about the absorption of energy.
Every day on earth, there are hundreds of earthquakes so small they are never felt, and probably thousands so small they don't even register.
An ad hom? I don't "expect" much from anyone but I find it surprising that you would resort to such.
I expected much better from you, Phage.
You didn't answer my question. Isn't conduction the transfer of thermal (kinetic) energy? Didn't you say previously that such an effect is not worth considering in the atmosphere? How does an atmosphere dominated by nitrogen get heated by sunlight?
A method to try and discredit facts shown... apparently. See above.
edit on 6/25/2013 by Phage because: (no reason given)
reply to post by Phage
Yes, I understand. Relevancy is in the eye of the beholder.
No, simply an expression of disappointment. An ad hominem argument would be poised to discredit the arguments based on the individual presenting them. The arguments you give need no such assistance.
See the response above.
And I am not going to.
You know perfectly well that you are twisting my responses around in order to discredit my original position, and attempting to confuse the issues.
I engaged you so heartily because I expected you to present relevant facts to back up your position... and you did once with the report of Bering Strait warming. I responded with an acceptance of your points after looking over the paper. But when you try to ignore the speed at which CO2 re-emits energy in order to try and change the subject to kinetic energy instead of radiation energy... when you pick and choose relevance based not on numbers but on which is beneficial to your argument... well, thank you for the debate.
TheRedneck
Slight yes, irrelevant no.
Now that is irrelevant.
Yes, I understand. Relevancy is in the eye of the beholder.
An ad hom?
No, simply an expression of disappointment. An ad hominem argument would be poised to discredit the arguments based on the individual presenting them. The arguments you give need no such assistance.
See the response above.
You didn't answer my question.
And I am not going to.
You know perfectly well that you are twisting my responses around in order to discredit my original position, and attempting to confuse the issues.
I engaged you so heartily because I expected you to present relevant facts to back up your position... and you did once with the report of Bering Strait warming. I responded with an acceptance of your points after looking over the paper. But when you try to ignore the speed at which CO2 re-emits energy in order to try and change the subject to kinetic energy instead of radiation energy... when you pick and choose relevance based not on numbers but on which is beneficial to your argument... well, thank you for the debate.
TheRedneck
new topics
-
Late Night with the Devil - a really good unusual modern horror film.
Movies: 1 hours ago -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 2 hours ago -
The Good News According to Jesus - Episode 1
Religion, Faith, And Theology: 4 hours ago -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three: 6 hours ago -
Bobiverse
Fantasy & Science Fiction: 9 hours ago -
Florida man's trip overseas ends in shock over $143,000 T-Mobile phone bill
Social Issues and Civil Unrest: 9 hours ago -
Former Labour minister Frank Field dies aged 81
People: 11 hours ago
top topics
-
Florida man's trip overseas ends in shock over $143,000 T-Mobile phone bill
Social Issues and Civil Unrest: 9 hours ago, 8 flags -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs: 13 hours ago, 7 flags -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 2 hours ago, 7 flags -
This is our Story
General Entertainment: 16 hours ago, 4 flags -
Former Labour minister Frank Field dies aged 81
People: 11 hours ago, 4 flags -
Bobiverse
Fantasy & Science Fiction: 9 hours ago, 3 flags -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three: 6 hours ago, 2 flags -
Late Night with the Devil - a really good unusual modern horror film.
Movies: 1 hours ago, 2 flags -
The Good News According to Jesus - Episode 1
Religion, Faith, And Theology: 4 hours ago, 0 flags
active topics
-
President BIDEN Vows to Make Americans Pay More Federal Taxes in 2025 - Political Suicide.
2024 Elections • 96 • : CriticalStinker -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest • 8 • : lordcomac -
British TV Presenter Refuses To Use Guest's Preferred Pronouns
Education and Media • 140 • : Annee -
Florida man's trip overseas ends in shock over $143,000 T-Mobile phone bill
Social Issues and Civil Unrest • 16 • : grey580 -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 22 • : RickyD -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs • 35 • : Consvoli -
Late Night with the Devil - a really good unusual modern horror film.
Movies • 1 • : DAVID64 -
Ditching physical money
History • 18 • : annonentity -
Lawsuit Seeks to ‘Ban the Jab’ in Florida
Diseases and Pandemics • 32 • : SchrodingersRat -
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest • 279 • : KrustyKrab

