It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
false DMCA copyright infringement takedowns filed by Mark Gray.
Those apollogists try everything to hide the truth.
It looks very very silly to me trying things like that.
Way to go Jarrah !!
edit on 19-4-2014 by webstra because: (no reason given)
Like one of the reactions on youtube : " It's only just a matter of time Jarrah, and everyone in the world will realize the truth."
edit on
19-4-2014 by webstra because: (no reason given)
originally posted by: Rob48
a reply to: FoosM
We as skeptics simply have to keep reminding as many people as we can to think critically about
what is being presented as fact, scenarios to manipulate us out of the freedoms, wealth and property that
we have fought and worked hard for. And sometimes even our lives.
You really don't understand what a skeptic is, do you? A skeptic is somebody who doesn't take wild claims and crackpot theories at face value but instead tests them and evaluates them based on evidence.
Yes, exactly.
Thats why I am a skeptic of Apollo and you are not.
You seem to be OK with the wild claim of 8 successful trips to the moon with no loss of life.
LOL. A feat never repeated in our highly technical times, after 40 years.
originally posted by: FoosM
WARNING: LONG POST. The important bit is in bold below.
Let's go back to this video, Foos. At last, some proper hard claims to test!
As you claim you are such a skeptic, no doubt you will have checked Jarrah's maths when he says that the PLSS water capacity would not be sufficient for the claimed cooling. (From about the 35:00 mark in the video)
If so, I suggest you check your working again, because he's totally wrong. My degree is in chemistry and although I no longer work in science, I can still remember about enthalpies and latent heats of evaporation and dull stuff like that (I hated phys chem!) and this is a relatively simple claim to test.
So, the issue Jarrah is disputing is that the PLSS could not provide cooling at a rate of 1600BTU per hour for a duration of four hours (or eight hours for the J-type missions). The details of the PLSS, which Jarrah only provides snippets of that are panned too fast to read properly, are here: www.hq.nasa.gov...
For a four-hour EVA the suits contained 3.9 litres of feedwater for cooling. For eight hours they carried 5.2 litres. Jarrah even quotes these numbers in his video.
Now, as you will know, the heat was carried away from the PLSS by sublimation into space.
The latent heat of sublimation for ice at 0 degrees C is 2.834 x 10^6 J/kg. Cite.
Apologies for the jargon, but if you're not a chemist I will add a quick layman's explanation here. If you know about these things then skip ahead beyond this part.
------------
To turn a piece of ice into water, say, you have to apply heat to it. That's because liquid water is at a higher energy than solid ice: the molecules are moving around all over the place, so water can flow, rather than being fixed in place like ice. To go from liquid water to water vapour, you have to apply even more energy, because a gas is even more energetic than a liquid - the molecules have enough energy that they are no longer confined by gravity to sit in their container but can diffuse out into the surroundings.
The amount of energy you need to put into a kilogram of water to change it from one of a lower-energy phase to a higher-energy phase (or, conversely, the energy released when it goes from a hgh-energy phase to a low-energy one) is called the "latent heat" of that phase change.
On the moon, in a vacuum, ice doesn't go through the liquid phase, because there is no atmospheric pressure: the ice can "boil off" straight into water vapour. That process is called sublimation.
The amount of energy it takes to turn a piece of ice that is already right at freezing point, into water vapour, is called the latent heat of sublimation.
So, each kilogram (i.e. litre) of water that is sublimating from the PLSS is carrying away 2.834 megajoules (MJ) of energy.
Sorry about the lengthy explanation, but I wanted to be sure you know what these figures mean.
------------
Now concentrate: here comes the maths bit:
The claimed cooling capacity of the PLSS is 1600 BTU per hour.
1 BTU = 1055 joules.
1600 BTU = 1600 x 1055 = 1688000 joules, or 1.688 MJ.
Latent heat of sublimation of water = 2.834 MJ kg^-1
Volume of water required to carry away 1.688 MJ of energy = 1.688 MJ / 2.834 MJ kg^-1 = 0.596kg.
So to carry away one hour's worth of heat, you need a fraction under 600ml of water. That's just over a pint.
For a four-hour EVA mission, they would have required 0.6 x 4 = 2.4 litres of water. They actually carried 3.9 litres. That's a pretty decent margin for error.
For an eight-hour EVA mission, they would have required 0.6 x 8 = 4.8 litres of water. They actually carried 5.2 litres. I guess they were confident that it worked as planned by then!
See, Foos, THAT is what being a skeptic is about. Checking figures. Jarrah White apparently took René's figures at face value without checking them at all. You apparently took Jarrah's video at face value without checking it at all. I am a skeptic, so I checked the maths, and surprise surprise it is utter moonshine (pun intended). If Jarrah is lying about this, then what else is he lying about?
If you disagree with my figures, please show me where I went wrong.
At 38:27, Jarrah says "In all the pro-NASA websites I have read, not a single one goes into detail about this colossal problem". Perhaps somebody could direct him here?
edit on 19-4-2014 by Rob48 because: Jarrah is an utter charlatan
a reply to: webstra
The "hoax evidence" that was presented here from that video Better Eight than Never was the idea that we should have seen visible clouds of water being expelled.
That evidence has already been debunked here. The water was not expelled in liquid form, but was sublimated away. Sublimation causes the H2O to be in a gaseous state (vapor), and the gaseous state of water in invisible. Liquid water cannot exist in a vacuum (well -- it can for very short times) because due to the lack of atmoepheric pressure, the water would sublimate away into its gas state.
...and water vapor is invisible.
Before you say "but I can see clouds", it should be pointed out to you that visible clouds are not H2O in a gaseous state -- visible clouds are made of liquid or frozen water droplets -- not gaseous water. There is water in its gaseous state (water vapor) in the entire atmosphere. When you see blue skies, you are looking through gaseous water/water vapor. When you see a visible cloud, you are seeing droplets of water in its liquid or frozen state.
The "hoax evidence" that was presented here from that video Better Eight than Never was the idea that we should have seen visible clouds of water being expelled.
That evidence has already been debunked here. The water was not expelled in liquid form, but was sublimated away. Sublimation causes the H2O to be in a gaseous state (vapor), and the gaseous state of water in invisible. Liquid water cannot exist in a vacuum (well -- it can for very short times) because due to the lack of atmoepheric pressure, the water would sublimate away into its gas state.
...and water vapor is invisible.
Before you say "but I can see clouds", it should be pointed out to you that visible clouds are not H2O in a gaseous state -- visible clouds are made of liquid or frozen water droplets -- not gaseous water. There is water in its gaseous state (water vapor) in the entire atmosphere. When you see blue skies, you are looking through gaseous water/water vapor. When you see a visible cloud, you are seeing droplets of water in its liquid or frozen state.
edit on 4/19/2014 by Soylent Green Is People because: (no reason given)
originally posted by: FoosM
Thats why I am a skeptic of Apollo and you are not.
But you are not a skeptic. You just swallow whole what people like Jarrah and René say without applying any sort of sanity filter. A skeptic doubts what he is told and tests the claims.
Read my long post above. I did the maths: Jarrah is talking nonsense. You are not a skeptic, because you did not check what he said, you just took it at face value.
I did the maths, based on the science, and the maths says NASA is right and Jarrah is wrong. Don't you just hate it when that happens? And it just keeps happening.

edit on 19-4-2014 by Rob48 because: added appropriate image
Thanks for the math.
But let me ask you this question.
Was any of the water used for drinking?
But let me ask you this question.
Was any of the water used for drinking?
originally posted by: FoosM
Thanks for the math.
But let me ask you this question.
Was any of the water used for drinking?
No. Do you really think they would design a system where if you got a bit thirsty and drank too much water you would reduce the duration of cooling capability? The astronauts wore a bag of drinking water beneath their suits. A bit like the Camelbak I wear when I go biking or skiing.
See here: www.hq.nasa.gov...
In-suit Drinking Device (ISDD) - The insuit drinking device ( Figure 2-26, below ) provides approximately 32 ounces of potable water within the PGA during lunar surface extravehicular activities. The ISDD consists of a flexible film bag with an inlet valve for filling and an outlet tube and tilt valve for drinking. The bag is attached between the PGA bladder and liner at the neck ring by means of hook-and-pile Velcro. The bag is filled with potable water from the spacecraft water system by means of the water-dispenser/fire-extinguisher.
edit on 19-4-2014 by Rob48
because: Added quote from link
originally posted by: Rob48
originally posted by: FoosM
Thanks for the math.
But let me ask you this question.
Was any of the water used for drinking?
No. Do you really think they would design a system where if you got a bit thirsty and drank too much water you would reduce the duration of cooling capability? The astronauts wore a bag of drinking water beneath their suits. A bit like the Camelbak I wear when I go biking or skiing.
See here: www.hq.nasa.gov...
In-suit Drinking Device (ISDD) - The insuit drinking device ( Figure 2-26, below ) provides approximately 32 ounces of potable water within the PGA during lunar surface extravehicular activities. The ISDD consists of a flexible film bag with an inlet valve for filling and an outlet tube and tilt valve for drinking. The bag is attached between the PGA bladder and liner at the neck ring by means of hook-and-pile Velcro. The bag is filled with potable water from the spacecraft water system by means of the water-dispenser/fire-extinguisher.
Ok so, how was this drink water kept cool in the sunlight? Or warm enough in the shade?
And, your calculations take in consideration what, the water from Liquid Cooling Garment (LCG) ?
Does it take in consideration anything else that had to be cooled?
originally posted by: FoosM
Ok so, how was this drink water kept cool in the sunlight? Or warm enough in the shade?
Did you understand what I posted? The drink bag was inside the suit next to the astronaut's body. It was kept at the right temperature, like everything inside the suit, by the PLSS that we are discussing! I imagine the water was probably round about body temperature if it was inside the suit, so it may not have been 100% cool and refreshing, but I think most of us would put up with drinking water that isn't fridge cold for a few hours if we were walking on the moon.
And, your calculations take in consideration what, the water from Liquid Cooling Garment (LCG) ?
Does it take in consideration anything else that had to be cooled?
Funny how you ask me all these questions, but apparently you didn't think to question any of what Jarrah White was spouting in his video. Why is everything he says taken on trust, but everything I say has to be constantly picked over?
In any case, your questions show that you haven't really read what I posted. I know there was a lot of it, but I spent a while doing the sums so at least do me the courtesy of reading what I wrote.
My calculations are based on the volume of water that was available to be sublimated into space from the PLSS, that is the cooling feedwater. The water flowing through the LCG was a separate system, whose only connection with the feedwater was via a heat exchanger. It was not consumed, it was just a constantly recirculating flow, like the coolant circulating through the back of your fridge. I haven't been able to find an exact figure for the volume of water flowing through the LCG itself, but this source gives the fully charged weight (i.e. garment plus water) as 2.09kg, so it cannot have been a huge volume. Even if we assumed that 90% of the weight of the charged LGC was water (which it obviously wasn't) then that would still be less than one of Jarrah's milk bottles! In any case, as I said, it is irrelevant to the actual water consumption because it was not being consumed!
From the link I posted:
Water circulated through the Liquid Cooling Garment (LCG) also flows through the heat exchanger where it gives up heat to a separate supply of cooling feedwater.
What do you mean by "Does it take in consideration anything else that had to be cooled?" The PLSS was only cooling the astronaut's suit. It was designed to have a cooling capacity of 1600 BTU per hour. Jarrah is not disputing the 1600 BTU per hour, he just claims that to have this cooling capacity it would require "23.78 litres of water" for four hours of cooling. I have demonstrated that it actually requires 2.4 litres of water. Notice anything? One-tenth, to within the rounding that I used. Looks like Jarrah - or rather René - dropped a decimal point somewhere? Hardly surprising when he used such a horrendous mish-mash of units in his working. There's a reason we use SI units in science!
Another mystery: why at 37:08 does Jarrah say "23.78 litres" when the calculations on screen (which are presented with no explanation, and seemingly start off by adding a random number to the 1600BTU figure!) conclude with a figure of 14.576kg,or 32lb? One kilogram of water is 1 litre.

Was 14.576 litres not big enough for Jarrah, so he added another 63% for luck? Does he think that when confronted with a wall of numbers, people will just glaze over and not pay attention to what they say?
Maybe if he spent more time with his calculator and less time buying gallons of milk, he might have spotted his error!
edit on 19-4-2014
by Rob48 because: fixed broken link coding
originally posted by: Rob48
originally posted by: FoosM
Ok so, how was this drink water kept cool in the sunlight? Or warm enough in the shade?
Did you understand what I posted? The drink bag was inside the suit next to the astronaut's body.
You mean beneath the outer suit, so not next the body, right. Or was it in the helmet?
And, your calculations take in consideration what, the water from Liquid Cooling Garment (LCG) ?
Does it take in consideration anything else that had to be cooled?
Your questions show that you haven't really read what I posted. I know there was a lot of it, but I spent a while doing the sums so at least do me the courtesy of reading what I wrote.
My calculations are based on the volume of water that was available to be sublimated into space, that is the cooling feedwater. The water flowing through the LCG was a separate, closed system, whose only connection with the feedwater was via a heat exchanger. It was not consumed, it was just a constantly recirculating flow, like the coolant circulating through the back of your fridge. From the link I posted:
Thats not what I was asking, maybe you need to comprehend my questions better.
What do you mean by "Does it take in consideration anything else that had to be cooled?"
Ah... now we are getting somewhere.
The PLSS was only cooling the astronaut's suit. It was designed to have a cooling capacity of 1600 BTU per hour. Jarrah is not disputing the 1600 BTU per hour,
Who is?
But getting back to my question. What heat was the suit actually cooling?
Was it cooling the batteries, etc?
Was it cooling the effects of solar radiation?
Was it cooling the heat exerted from the astronauts themselves?
Or all the above?
How many BTUs is that in total?
For example, humans generate about per hour
250 BTU's Sleeping
1040 BTU's Walking (3mph)
1600 BTU's Factory work (heavy)
1800 Exercise (heavy) -
I would think the astronauts, having to move in those suits, would be
exerting between Factory Work and Exercise conditions. No?
But that can't be the only heat the PLSS had to deal with.
So the solar heat flux is calculated to 10,000 Btu per hour.
How much of that heat did the Astronaut's suit limit?
How much of that heat did the Astronaut's suit limit?
originally posted by: FoosM
originally posted by: wmd_2008
a reply to: FoosM
Ahmmm!!!
STILL WAITING
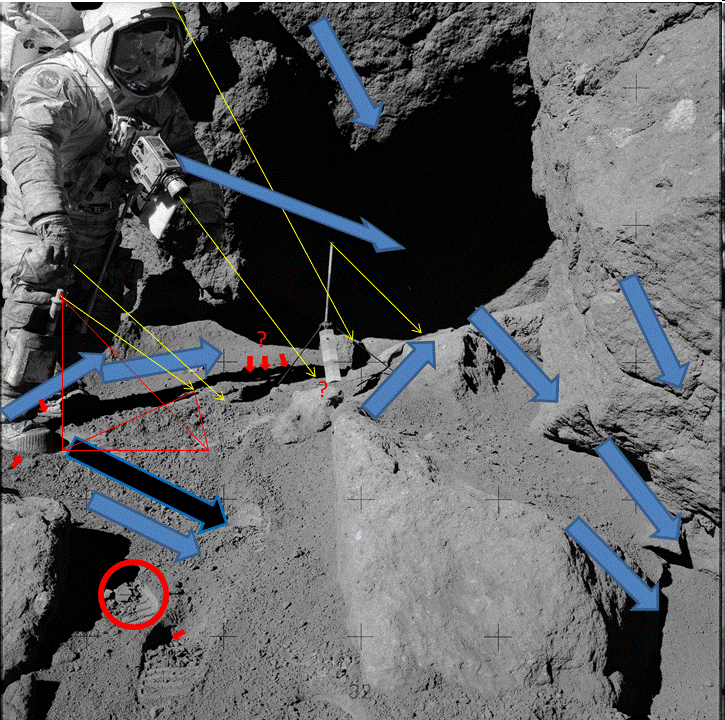
Man, what are you still waiting for, not enough arrows for you to understand whats going on??
I did it for you guys.
Yes and the arrows show that you don't understand what junior school kids would understand, so please explain how you think your arrows are correct I can supply some crayons if you need them
originally posted by: FoosM
But getting back to my question. What heat was the suit actually cooling?
Was it cooling the batteries, etc?
Was it cooling the effects of solar radiation?
Was it cooling the heat exerted from the astronauts themselves?
Or all the above?
How many BTUs is that in total?
For example, humans generate about per hour
250 BTU's Sleeping
1040 BTU's Walking (3mph)
1600 BTU's Factory work (heavy)
1800 Exercise (heavy) -
I would think the astronauts, having to move in those suits, would be
exerting between Factory Work and Exercise conditions. No?
But that can't be the only heat the PLSS had to deal with.
I take it those figures are under Earth's gravity
originally posted by: wmd_2008
originally posted by: webstra
originally posted by: FoosM
So the solar heat flux is calculated to 10,000 Btu per hour.
How much of that heat did the Astronaut's suit limit?
Cool quote Foosm :-)
It would be cool if you understood it
People who understand that the moonlanding was a fraud do understand a lot more then you can ever imagine wmd_2008.....i'm afraid.
originally posted by: FoosM
You mean beneath the outer suit, so not next the body, right. Or was it in the helmet?
It is between the PGA bladder and the liner, i.e. inside the pressurised suit environment. Seriously Foos, all this stuff is in the public domain. Why do you not go and find it for yourself?
But getting back to my question. What heat was the suit actually cooling?
Was it cooling the batteries, etc?
Was it cooling the effects of solar radiation?
Was it cooling the heat exerted from the astronauts themselves?
Or all the above?
How many BTUs is that in total?
The electrical system (batteries) didn't generate that much heat. The nominal power ratings for the electrical systems in the suit were as follows: [1]
Pump: 8.4 W
Fan: 21.8 W
EVCS: 10.9W.
Total = 41.1 watts, or 140 BTU per hour, even if all three systems were running all the time.
So the solar heat flux is calculated to 10,000 Btu per hour.
How much of that heat did the Astronaut's suit limit?
You're the skeptic - you tell me! Why do you think the suits were white? Answer, white reflects a large proportion of that heat.
As it happens, I have a cite here. In full sunlight, the maximum solar radiation IN FULL SUNLIGHT absorbed by the space suits was calculated as 203 watts, or 692 BTU per hour. [2]
And not all of that heat penetrated into the inner suit. Because of the insulation between the outer and inner layers, heat leakage into or out of the suits was limited to 250 BTU/hr (into the suit, during the lunar day) and 350 BTU/hr (out of the suit, during the lunar night). [3]
For example, humans generate about per hour
250 BTU's Sleeping
1040 BTU's Walking (3mph)
1600 BTU's Factory work (heavy)
1800 Exercise (heavy) -
I would think the astronauts, having to move in those suits, would be
exerting between Factory Work and Exercise conditions. No?
Those figures are based on EARTH GRAVITY! The astronauts' body weight was one sixth of that on the Earth, so moving around expends less energy. With that in mind, the PLSS design which was based on a nominal metabolic rate of 1200 BTU per hour [1] looks quite generous.
So let's do the maths:
Heat gains:
Electrical system: 140 BTU/hr
Solar gain: 250 BTU/hr
Astronaut metabolic rate: 1200 BTU/hr
Total: 1590 BTU/hr
Heat loss: 1600 BTU/hr
Pretty close! Now you might think that is not much margin for error (I must admit I was surprised the figures came out as close as that) but remember, those gains are assuming that (a) the astronaut is always in full sun, (b) he doesn't rest at all during the EVA. Even then the PLSS can cope.
Remember also that the suit temperature was being monitored via telemetry all the time. If heat was building up excessively, Mission Control could tell the astronauts to rest up for a while or seek shade. They weren't moving at full tilt all the time. Could they theoretically have overheated if they'd run around in full sun for four hours straight, such that they were generating more than 1200 BTU/hr? Sure. But they didn't do that.
Once again - even Jarrah is not trying to claim that 1600 BTU/hr is insufficent. He is claiming that the volume of water in the PLSS was insufficient to provide that cooling. As I have shown, that is totally incorrect.
Foos, all the information is out there. If you doubt something, DO THE MATHS. That way you might become a true skeptic, and realise that Jarrah & co are not worthy of your time. Do you begin to see that? If he lies about something as basic about 2.4 litres versus 23 litres, why should you trust everything else he says?
Citations:
[1] Apollo Operations Handbook: Extra Vehicular Mobility Unit www.lpi.usra.edu...
[2] D. R. Pitts & L. E. Sissom, "1000 Solved Problems In Heat Transfer" (McGraw Hill, 1991)
[3] www.apollosaturn.com...
edit on 19-4-2014 by Rob48 because: added citation
new topics
-
Any one suspicious of fever promotions events, major investor Goldman Sachs card only.
The Gray Area: 1 hours ago -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology: 6 hours ago -
Electrical tricks for saving money
Education and Media: 9 hours ago -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 10 hours ago -
Sunak spinning the sickness figures
Other Current Events: 11 hours ago -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues: 11 hours ago
top topics
-
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 10 hours ago, 9 flags -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 14 hours ago, 8 flags -
Electrical tricks for saving money
Education and Media: 9 hours ago, 4 flags -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues: 11 hours ago, 3 flags -
Sunak spinning the sickness figures
Other Current Events: 11 hours ago, 3 flags -
Late Night with the Devil - a really good unusual modern horror film.
Movies: 13 hours ago, 2 flags -
Any one suspicious of fever promotions events, major investor Goldman Sachs card only.
The Gray Area: 1 hours ago, 2 flags -
The Good News According to Jesus - Episode 1
Religion, Faith, And Theology: 16 hours ago, 1 flags -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology: 6 hours ago, 0 flags
active topics
-
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues • 22 • : lincolnriley -
The Acronym Game .. Pt.3
General Chit Chat • 7747 • : F2d5thCavv2 -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 37 • : FlyersFan -
Any one suspicious of fever promotions events, major investor Goldman Sachs card only.
The Gray Area • 3 • : seekshelter -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology • 13 • : andy06shake -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 662 • : F2d5thCavv2 -
SETI chief says US has no evidence for alien technology. 'And we never have'
Aliens and UFOs • 61 • : andy06shake -
The Reality of the Laser
Military Projects • 47 • : F2d5thCavv2 -
Definitive 9.11 Pentagon EVIDENCE.
9/11 Conspiracies • 423 • : Lazy88 -
Election Year 2024 - Interesting Election-Related Tidbits as They Happen.
2024 Elections • 73 • : Threadbarer