It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
Originally posted by Legion2024
reply to post by mainidh
What are they trying to hide...? I think the fact is they just simply don't know.
I say lets send a probe right in to a sunspot and find out
Oo I broke your sarcasm detector
Probe the sun.. Hrmm, well I think it's the Russians that are planning on landing a satellite onto an asteroid in 2029. Maybe they can drop it off on the way back to bomb the sun and find water deposits?!
Worked for the moon!!
*sheds tear for the moon people killed in that terrible thing*
reply to post by BohemianBrim
Its actually pretty believable if you understand basic chemistry and physics.
Not to mention, if you actually do any reading on the matter, it pretty well explains how it happens.
Its actually pretty believable if you understand basic chemistry and physics.
Not to mention, if you actually do any reading on the matter, it pretty well explains how it happens.
Originally posted by mainidh
Originally posted by Hawking
So parts of these sunspots are less than 100C or 212F?
Really?
Why is no one reading the article? It's all in the article... It's water vapor for a start..
Agreed. Frustrating as hell to see basic questions asked that are addressed in the article.
Anybody else think of this???
Maybe using the sun/singularity as a Stargate isn't such a crazy idea after all considering its that cool
Maybe using the sun/singularity as a Stargate isn't such a crazy idea after all considering its that cool
edit on 17-6-2012 by TritonTaranis because: (no reason given)
reply to post by chr0naut
??
The sun is continuously fusing heavier and heavier elements, one of them being oxygen. So there will always be a plentiful amount of hydrogen and oxygen on the sun (for the time being) to create water.
??
The sun is continuously fusing heavier and heavier elements, one of them being oxygen. So there will always be a plentiful amount of hydrogen and oxygen on the sun (for the time being) to create water.
double post
edit on 6/17/2012 by VonDoomen because: (no reason given)
reply to post by Hawking
I didnt read the article but I doubt its, LIQUID water, but more like hydrogen / oxygen bonds
I didnt read the article but I doubt its, LIQUID water, but more like hydrogen / oxygen bonds
Well, so we can ask the Sun to send water to quench those stubborn fires around the country. Mother Earth isn't going to help us with our fires
anymore. She said she's going to destroy our economy for disrespecting her.
reply to post by tauristercus
There is also the possibility of these ionic or polar molecules to be thrown around by magnetic fields. So these water molecules may not be directly on the "surface" of the sun. These water molecules may also be buried deep with clouds of matter that they mentioned. And also dont forget that comets and meteors that crash into the sun can also be carrying water as well.
There is also the possibility of these ionic or polar molecules to be thrown around by magnetic fields. So these water molecules may not be directly on the "surface" of the sun. These water molecules may also be buried deep with clouds of matter that they mentioned. And also dont forget that comets and meteors that crash into the sun can also be carrying water as well.
Originally posted by stanguilles7
Originally posted by mainidh
Originally posted by Hawking
So parts of these sunspots are less than 100C or 212F?
Really?
Why is no one reading the article? It's all in the article... It's water vapor for a start..
Agreed. Frustrating as hell to see basic questions asked that are addressed in the article.
Well whatever it is they're looking at isn't actually water then, is it?
It MIGHT be steam...maybe?
And we've got a thread titled "Water found on the sun."
In no practical sense whatsoever is that water
What the hell guys.....
When were there ever plants on the sun to produce oxygen for hydrogen to bond to for even a second to create steam???????
WHY is this being discussed???
Space is a vacuum - NO OXYGEN. PERIOD.
When were there ever plants on the sun to produce oxygen for hydrogen to bond to for even a second to create steam???????
WHY is this being discussed???
Space is a vacuum - NO OXYGEN. PERIOD.
reply to post by Hawking
It is the title of the article on the University site..
And we've got a thread titled "Water found on the sun." In no practical sense whatsoever is that water
It is the title of the article on the University site..
1995-05-25 00:00:00
Water found on the sun
University of Waterloo News Bureau
EMBARGOED FOR RELEASE: 6 p.m. EDT May 25, 1995
edit on 17-6-2012 by maxella1 because: (no reason given)
Originally posted by BohemianBrim
i dont think i can believe that.
AMEN. I'm no chemist but I know that water boils when at a certain temperature heat. When the heat is very strong, it starts to evaporate. I'm sure the sun is way way WAY hotter than that. Water would evaporate i think instantaneously on the sun. Even on the surface.
Water vapor separates to hydrogen and oxygen at around 2800C to 3200C+. When hydrogen is anywhere near a flame or spark it will ignite
instantaneously. Pure oxygen is also flammable.
Therefore their is NO hydrogen supply on the sun. It would instantly be ignited. you cannot "store" a flammable gas in a big ball of flames. Now their may be hydrogen or water vapor being sucked into the sun, but once it gets there it will be ignited.
Therefore their is NO hydrogen supply on the sun. It would instantly be ignited. you cannot "store" a flammable gas in a big ball of flames. Now their may be hydrogen or water vapor being sucked into the sun, but once it gets there it will be ignited.
reply to post by tauristercus
Well Water [snide humor] loo University did investigate it, so it must be true.
Well Water [snide humor] loo University did investigate it, so it must be true.
edit on 17-6-2012 by Manhater because: (no reason given)
Originally posted by Manhater
Originally posted by BohemianBrim
i dont think i can believe that.
Yeah, seriously, I would of found it first.
And with how hot the sun is, it would like totally evaporate.
edit on 17-6-2012 by Manhater because: (no reason given)
he said it was vapor.
do you just read threat titles and skip the content in the post?
reply to post by prevenge
Well duh, water does turn into vapor the last time I checked.
Have you ever tried boiling it?
I burnt a pot of water.
I know.
Well duh, water does turn into vapor the last time I checked.
Have you ever tried boiling it?
I burnt a pot of water.
I know.
edit on 17-6-2012 by Manhater because: (no reason given)
how can this not be true? a scientist said this so who are you guys to say its not? he's a cientist ffs. remember the neutrino? they apparently
grabbed one of these thing, so tiny that itll just slip through the cracks of an atom, and threw it hundred of miles away and caught it again with
their bare hand. if they can do this, what makes you think they cant say theres water on the sun? cmon, wake up guys, dont be doubting superior
intellects like that.
Well, this is where my old school studies have left me in awe again. When I was attending school, we were pretty much taught nothing existed on the
sun...especially water. Now I know the article stated vapor/steam, but my ingrained learning says "Shut The Front Door!!!" anyway.
I would love it if they could find a way to study the sun more or more closely. Something we have stared at, depended on, bathed in, and in some instances worshiped.... is still shrouded in tons of mystery.
I love learning these things! S+F OP for teaching me something new today!
I would love it if they could find a way to study the sun more or more closely. Something we have stared at, depended on, bathed in, and in some instances worshiped.... is still shrouded in tons of mystery.
I love learning these things! S+F OP for teaching me something new today!
Originally posted by VonDoomen
reply to post by tauristercus
So these water molecules may not be directly on the "surface" of the sun. These water molecules may also be buried deep with clouds of matter that they mentioned
I'd like to see a molecule of water survive (either on the surface or above it) when bombarded by the sun's intense ultraviolet output.
If water molecules are easily broken in the earth's atmosphere by UV, then large quantities of water molecules in the sun's vicinity doesn't stand a snowball's chance in hell of remaining in one piece.
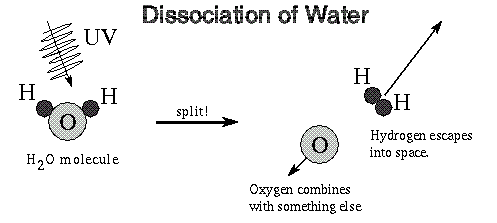
The combination of extremely high temps, radiation (alpha, beta, gamma) and UV will all combine to shred individual water molecules into it's constituents of hydrogen and oxygen.
Are these scientists claiming some "new and exotic" physics to allow a water molecule to survive in such an extremely hostile environment ?
Also, if as they claim, a molecule of water CAN survive ... then what about other molecules such as carbon dioxide, ammonia, etc ? Can they exist as well ?
new topics
-
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies: 1 hours ago -
Hurt my hip; should I go see a Doctor
General Chit Chat: 2 hours ago -
Israel attacking Iran again.
Middle East Issues: 3 hours ago -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 3 hours ago -
When an Angel gets his or her wings
Religion, Faith, And Theology: 4 hours ago -
Comparing the theology of Paul and Hebrews
Religion, Faith, And Theology: 5 hours ago -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 6 hours ago -
Boston Dynamics say Farewell to Atlas
Science & Technology: 6 hours ago -
I hate dreaming
Rant: 7 hours ago -
Man sets himself on fire outside Donald Trump trial
Mainstream News: 9 hours ago
top topics
-
The Democrats Take Control the House - Look what happened while you were sleeping
US Political Madness: 10 hours ago, 18 flags -
In an Historic First, In N Out Burger Permanently Closes a Location
Mainstream News: 12 hours ago, 16 flags -
A man of the people
Medical Issues & Conspiracies: 17 hours ago, 11 flags -
Man sets himself on fire outside Donald Trump trial
Mainstream News: 9 hours ago, 9 flags -
Biden says little kids flip him the bird all the time.
Politicians & People: 9 hours ago, 9 flags -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 3 hours ago, 6 flags -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 6 hours ago, 6 flags -
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies: 1 hours ago, 6 flags -
Israel attacking Iran again.
Middle East Issues: 3 hours ago, 5 flags -
Boston Dynamics say Farewell to Atlas
Science & Technology: 6 hours ago, 4 flags
active topics
-
Israel attacking Iran again.
Middle East Issues • 27 • : KrustyKrab -
I hate dreaming
Rant • 7 • : TheMichiganSwampBuck -
MULTIPLE SKYMASTER MESSAGES GOING OUT
World War Three • 53 • : Zaphod58 -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media • 8 • : TheMichiganSwampBuck -
I Guess Cloud Seeding Works
Fragile Earth • 29 • : Justoneman -
Man sets himself on fire outside Donald Trump trial
Mainstream News • 40 • : Vermilion -
When an Angel gets his or her wings
Religion, Faith, And Theology • 5 • : randomuser2034 -
Anyone one else having Youtube problems
Computer Help • 11 • : charlyv -
The Democrats Take Control the House - Look what happened while you were sleeping
US Political Madness • 68 • : Mahogani -
Candidate TRUMP Now Has Crazy Judge JUAN MERCHAN After Him - The Stormy Daniels Hush-Money Case.
Political Conspiracies • 404 • : Zanti Misfit
