It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
Dummies Guide to GOLD bullion refining at home as a long term precious metal investment - made EASY
page: 1share:
As informed ATS members, I'm sure you're all perfectly aware of the extreme volatility in the current global financial market and even more aware of
how our money buys less and less and less as the years go by. What's also very apparent to those that watch the precious metals market e.g. silver
and gold, that contrary to the "value" of paper based currency which has been continuously decreasing, that the "value" of silver and gold has
been on a continual increase.
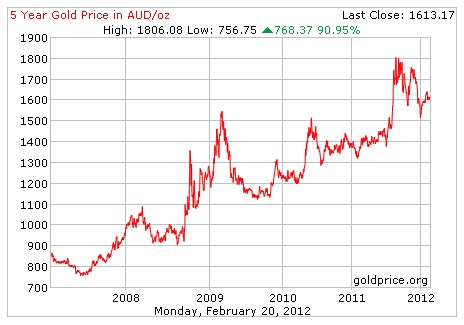
Here's a graph showing the huge gains gold has made over the last 5 years. If you'd been smart enough (or lucky enough) to invest in gold back then, today you'd be sitting on a very pretty profit, indeed !
Now over the last few years, I've been taking scrap, old, unwanted and broken items containing gold and refining them in such a way as to end up with essentially 99.9% gold bullion. Originally this was nothing more than a home hobby of sorts but with the value of gold bullion apparently on an increasing trend, what started out as just a hobby has now become a means of creating gold bullion as a long term investment. I get quite a kick in checking the gold markets and watching the value of my gold slowly rise
And so I figured that now would be a perfect opportunity to share my experience in gold bullion creation at home with other ATS members who may have an interest.
Not only will you end up being able to create 99.9% pure 24k gold bullion of your own, but you'll also see how ridiculously simple and easy the process is and that the majority of equipment is easily obtainable and very inexpensive.
In fact, over the years I've simplified the entire process so that anyone can do it. So why not read this tutorial and perhaps give it a go yourself.
Just to whet your appetite, here's a pic of a pure gold 24k button (8 gms) I made earlier ... and worth approx. $US447 at todays market rates ... hopefully next week will be worth a bit more

Before we get started, I need to provide a WARNING.
Even though in my previous silver thread I thought that I had issued sufficient warnings due to the fact that strong acids were being used in the process and that all due diligence, care and common sense was to be used at all times, there were still a few members who basically gave the impression that handling nitric acid was just so incredibly dangerous and should only be used by "trained professionals" and NOT for the average "Joe Blow".
However, I'd like to YET AGAIN stress that if proper precautions are taken, care is exercised and basic common sense used, then handling very small quantites of nitric (and in this tutorial, hydrochloric) acids is no more dangerous than many other potentially harmful chemicals normally found in the average kitchen, laundry or garage that we use everday. Chemicals such as strong bleach, drain "unblockers", swimming pool chemicals, ammonia, tree stump killers, car battery acid, etc ALL have the potential to cause serious harm if misused ... however, I'm sure that the average, level headed person is able to handle and use such "dangerous" chemicals without any harm whatsoever ... simply because they exercise care and common sense when using them.
So it is with the acids used in silver and gold refining ... care and common sense.
Still interested ?
Ok, let's start processing gold !
We need some basic equipment which consists of a plastic water spray bottle, a plastic spoon, a plastic "swizzle" stick, a plastic "turkey baster" pipette and a small plastic funnel.
All these items were purchased at one of those "cheap" stores for just a few dollars only.

We need a couple of small glass cylinders and in my case, I used some "empty" spice jars from the kitchen. At least that's what I told my wife
Also need a small glass bowl able to stand up to boiling water and that will allow one of the spice jars to be placed upright in it. The boiling water poured into the bowl will help heat the contents of the spice jar.

We will also need some cheap disposable plastic gloves such as the ones used for food handling.
We'll also need some coffee filters. Again, both of these items were purchased at the "cheap" store for a couple of dollars.

The next items that we'll need is a bottle of 68-70% strength Nitric acid and a bottle of 32% Hydrochloric acid. These are both readily obtainable from the majority of chemical supply companies which you'll have no problems in finding in your local yellow pages. A 500 ml bottle of each will be quite adequate.
The nitric acid is used to dissolve any other contaminating metals in the item containing gold such as copper, silver, etc. We need to remove such contaminants leaving behind relatively clean (but not yet pure) gold for final processing.
The hydrochloric acid will be used in conjunction with the nitric acid to dissolve and purify the gold after the other metal contaminants have been removed by the nitric acid.

At this point, it's time for another WARNING reminder, so PLEASE PAY ATTENTION.
Nitric and hydrochloric acids, by their nature are powerful corrosive acids and skin contact should be avoided. When working with them, you should ALWAYS be wearing those disposable plastic gloves that we purchased earlier. Eye protection would also be advisable.
Simple care, caution and common sense is all that's needed and you'll be perfectly safe. In all the years that I've been refining silver and gold for a hobby and using nitric and hydrochloric acids, not once has there been an issue or concern. If any is spilled on a surface, just immediately rinse off with plenty of plain, clean water.
Also, small quantities of nitrogen dioxide gas is produced so ALWAYS do the refining outside in the open air.
The motto is: "Be careful and be safe".
But let me reiterate one more time ... if you exercise common sense and basic care, this entire procedure is perfectly safe. Again, I've been doing this for quite a few years with NO mishaps whatsoever.
Moving right along ...
There are 2 additional items that we will require to process gold but weren't necessary for silver processing.
These items are Urea and Sodium Meta Bisulphite.
Urea is nothing more than plant fertilizer and can be found at almost all plant nurseries and large supermarkets with a garden section. A big bag is worth just a few dollars.

Sodium Meta Bisulphite, despite it's very scientific name, is very common and can be found in any store/shop that supplies home brewing kits/products so there should be no probs in locating it. SMB is used for sterilizing the home brew containers to kill any yeast. A small bag is all thats needed and costs just a few dollars.

So as you can see, processing pure gold requires just 3 additional items (hydrochloric acid, urea and SMB) more than was needed to process pure silver.
Silver processing = VERY easy
Gold processing = VERY easy (almost)
Continued next post ...
Here's a graph showing the huge gains gold has made over the last 5 years. If you'd been smart enough (or lucky enough) to invest in gold back then, today you'd be sitting on a very pretty profit, indeed !

Now over the last few years, I've been taking scrap, old, unwanted and broken items containing gold and refining them in such a way as to end up with essentially 99.9% gold bullion. Originally this was nothing more than a home hobby of sorts but with the value of gold bullion apparently on an increasing trend, what started out as just a hobby has now become a means of creating gold bullion as a long term investment. I get quite a kick in checking the gold markets and watching the value of my gold slowly rise
And so I figured that now would be a perfect opportunity to share my experience in gold bullion creation at home with other ATS members who may have an interest.
Not only will you end up being able to create 99.9% pure 24k gold bullion of your own, but you'll also see how ridiculously simple and easy the process is and that the majority of equipment is easily obtainable and very inexpensive.
In fact, over the years I've simplified the entire process so that anyone can do it. So why not read this tutorial and perhaps give it a go yourself.
Just to whet your appetite, here's a pic of a pure gold 24k button (8 gms) I made earlier ... and worth approx. $US447 at todays market rates ... hopefully next week will be worth a bit more

Before we get started, I need to provide a WARNING.
Even though in my previous silver thread I thought that I had issued sufficient warnings due to the fact that strong acids were being used in the process and that all due diligence, care and common sense was to be used at all times, there were still a few members who basically gave the impression that handling nitric acid was just so incredibly dangerous and should only be used by "trained professionals" and NOT for the average "Joe Blow".
However, I'd like to YET AGAIN stress that if proper precautions are taken, care is exercised and basic common sense used, then handling very small quantites of nitric (and in this tutorial, hydrochloric) acids is no more dangerous than many other potentially harmful chemicals normally found in the average kitchen, laundry or garage that we use everday. Chemicals such as strong bleach, drain "unblockers", swimming pool chemicals, ammonia, tree stump killers, car battery acid, etc ALL have the potential to cause serious harm if misused ... however, I'm sure that the average, level headed person is able to handle and use such "dangerous" chemicals without any harm whatsoever ... simply because they exercise care and common sense when using them.
So it is with the acids used in silver and gold refining ... care and common sense.
Still interested ?
Ok, let's start processing gold !
We need some basic equipment which consists of a plastic water spray bottle, a plastic spoon, a plastic "swizzle" stick, a plastic "turkey baster" pipette and a small plastic funnel.
All these items were purchased at one of those "cheap" stores for just a few dollars only.

We need a couple of small glass cylinders and in my case, I used some "empty" spice jars from the kitchen. At least that's what I told my wife
Also need a small glass bowl able to stand up to boiling water and that will allow one of the spice jars to be placed upright in it. The boiling water poured into the bowl will help heat the contents of the spice jar.

We will also need some cheap disposable plastic gloves such as the ones used for food handling.
We'll also need some coffee filters. Again, both of these items were purchased at the "cheap" store for a couple of dollars.

The next items that we'll need is a bottle of 68-70% strength Nitric acid and a bottle of 32% Hydrochloric acid. These are both readily obtainable from the majority of chemical supply companies which you'll have no problems in finding in your local yellow pages. A 500 ml bottle of each will be quite adequate.
The nitric acid is used to dissolve any other contaminating metals in the item containing gold such as copper, silver, etc. We need to remove such contaminants leaving behind relatively clean (but not yet pure) gold for final processing.
The hydrochloric acid will be used in conjunction with the nitric acid to dissolve and purify the gold after the other metal contaminants have been removed by the nitric acid.

At this point, it's time for another WARNING reminder, so PLEASE PAY ATTENTION.
Nitric and hydrochloric acids, by their nature are powerful corrosive acids and skin contact should be avoided. When working with them, you should ALWAYS be wearing those disposable plastic gloves that we purchased earlier. Eye protection would also be advisable.
Simple care, caution and common sense is all that's needed and you'll be perfectly safe. In all the years that I've been refining silver and gold for a hobby and using nitric and hydrochloric acids, not once has there been an issue or concern. If any is spilled on a surface, just immediately rinse off with plenty of plain, clean water.
Also, small quantities of nitrogen dioxide gas is produced so ALWAYS do the refining outside in the open air.
The motto is: "Be careful and be safe".
But let me reiterate one more time ... if you exercise common sense and basic care, this entire procedure is perfectly safe. Again, I've been doing this for quite a few years with NO mishaps whatsoever.
Moving right along ...
There are 2 additional items that we will require to process gold but weren't necessary for silver processing.
These items are Urea and Sodium Meta Bisulphite.
Urea is nothing more than plant fertilizer and can be found at almost all plant nurseries and large supermarkets with a garden section. A big bag is worth just a few dollars.

Sodium Meta Bisulphite, despite it's very scientific name, is very common and can be found in any store/shop that supplies home brewing kits/products so there should be no probs in locating it. SMB is used for sterilizing the home brew containers to kill any yeast. A small bag is all thats needed and costs just a few dollars.

So as you can see, processing pure gold requires just 3 additional items (hydrochloric acid, urea and SMB) more than was needed to process pure silver.
Silver processing = VERY easy
Gold processing = VERY easy (almost)
Continued next post ...
Continued from previous post ...
And to complete our refining "kit", we need someway of melting the raw gold into the shiny metal that people have lusted after for millenia
Thankfully, all we need to do is head to the nearest hardware store and get something along the lines of this badboy ... a propane powered torch. This is the one I use because it has a self-igniting switch built in, so no need to use matches to get it going. Also has a rotary button to control the strength of the flame. No problems whatsoever in finding one as they're a common handyman item and it easily melts silver and gold.

Well, thats it ... that's all the simple equipment that we need to start producing our very own 99.9% pure gold bullion and then watching it appreciate over time !
And as you can see, all of it is extremely easy to find.
As for the material containing gold that will be processed, sure you could use old bits of gold jewelry or anything else that you've got lying around with a reasonable gold content. But personally, I found the simplest way to get all my "raw gold" is in the form of "gold nuggets" purchased on eBay ... especially after having used up all my wife's gold jewelry ... just kidding Quite often you can get some really good deals.
As an example, last week I purchased a total of 10 gms of gold nuggets. Each nugget will usually contain 95% or higher amounts of gold with minor traces of impurities such as quartz grains. Sure beats trying to find them yourself and dig them out of the ground the hard way !
Here's 2 of them:

Ok, for the purpose of this tutorial, lets process some scrap gold that I've got lying around and recover the pure gold from it. The gold "button" has impurities so I'll use this tutorial to re-refine it. The pins and edge connectors came from 2 old computer motherboards and a few sticks of computer memory. There is gold to be found in computer parts but usually it's just a very thin coating ... still, gold is gold !

To dissolve the metal contaminants, we need to use a 50/50 mixture of nitric acid and water. The amount of nitric acid required will be approximately 20 mls mixed with approximately 20 mls of water. Using the plastic pipette, the water is added first followed by the nitric acid.

To help speed the reaction along, the entire glass tube is placed in a bath of boiling water. Periodically, as it cools, replace the bath water with fresh boiling water to keep the reaction going.
There is an immediate strong reaction as copper contaminants immediately start turning the liquid a blue colour.
Also notice the release of brown nitrogen dioxide gas. At this point, walk away from the reaction and do NOT inhale the gas. The reaction can proceed unattended until there is no more bubbling visible.

When the reaction finally stops, add an additional very small quantity (a few mls) of nitric. If bubbling starts up again, then not all the contaminant metals have been dissolved yet and once again, allow the process to continue on its own. If adding further nitric does NOT result in bubbling, then all metal contaminants will have been dissolved leaving behind relatively pure gold.
At this point, you can dilute the blue liquid with water, wait for the gold to settle to the bottom, then carefully pour most of the liquid into another container. Just be careful and don't pour away any of the gold !!
Add some more water to the settled gold to dilute even further any remaining liquid, then let the gold settle again, then carefully pour most of the liquid into another container ... again, DON'T pour away any of the gold.
After repeating the dilute/pour procedure a few times (basically "washing" the gold), we end up with something like this:

The above is the gold obtained from those computer motherboard pins and memory edges.
Note: If you use gold jewelry or any kind of gold/silver mixture, then chances are good that the blue liquid that you've been diluting/pouring off will contain reasonable amounts of dissolved silver. In that case, the silver can also be recovered by using the method I described in my earlier Dummies Guide to EASY silver bullion refining at home as a long term precious metal investment thread.
Having successfully removed almost all of the contaminants leaving the gold in an "almost" pure state, we're now going to process the gold one last time to remove any minute stubborn contaminants and end up with essentially 99.9% pure 24k gold ... woohoo !!
In this final step, we're going to do some "alchemy" ... just like the Ancients used to do !
Continued next post ...
And to complete our refining "kit", we need someway of melting the raw gold into the shiny metal that people have lusted after for millenia
Thankfully, all we need to do is head to the nearest hardware store and get something along the lines of this badboy ... a propane powered torch. This is the one I use because it has a self-igniting switch built in, so no need to use matches to get it going. Also has a rotary button to control the strength of the flame. No problems whatsoever in finding one as they're a common handyman item and it easily melts silver and gold.

Well, thats it ... that's all the simple equipment that we need to start producing our very own 99.9% pure gold bullion and then watching it appreciate over time !
And as you can see, all of it is extremely easy to find.
As for the material containing gold that will be processed, sure you could use old bits of gold jewelry or anything else that you've got lying around with a reasonable gold content. But personally, I found the simplest way to get all my "raw gold" is in the form of "gold nuggets" purchased on eBay ... especially after having used up all my wife's gold jewelry ... just kidding Quite often you can get some really good deals.
As an example, last week I purchased a total of 10 gms of gold nuggets. Each nugget will usually contain 95% or higher amounts of gold with minor traces of impurities such as quartz grains. Sure beats trying to find them yourself and dig them out of the ground the hard way !
Here's 2 of them:

Ok, for the purpose of this tutorial, lets process some scrap gold that I've got lying around and recover the pure gold from it. The gold "button" has impurities so I'll use this tutorial to re-refine it. The pins and edge connectors came from 2 old computer motherboards and a few sticks of computer memory. There is gold to be found in computer parts but usually it's just a very thin coating ... still, gold is gold !

To dissolve the metal contaminants, we need to use a 50/50 mixture of nitric acid and water. The amount of nitric acid required will be approximately 20 mls mixed with approximately 20 mls of water. Using the plastic pipette, the water is added first followed by the nitric acid.

To help speed the reaction along, the entire glass tube is placed in a bath of boiling water. Periodically, as it cools, replace the bath water with fresh boiling water to keep the reaction going.
There is an immediate strong reaction as copper contaminants immediately start turning the liquid a blue colour.
Also notice the release of brown nitrogen dioxide gas. At this point, walk away from the reaction and do NOT inhale the gas. The reaction can proceed unattended until there is no more bubbling visible.

When the reaction finally stops, add an additional very small quantity (a few mls) of nitric. If bubbling starts up again, then not all the contaminant metals have been dissolved yet and once again, allow the process to continue on its own. If adding further nitric does NOT result in bubbling, then all metal contaminants will have been dissolved leaving behind relatively pure gold.
At this point, you can dilute the blue liquid with water, wait for the gold to settle to the bottom, then carefully pour most of the liquid into another container. Just be careful and don't pour away any of the gold !!
Add some more water to the settled gold to dilute even further any remaining liquid, then let the gold settle again, then carefully pour most of the liquid into another container ... again, DON'T pour away any of the gold.
After repeating the dilute/pour procedure a few times (basically "washing" the gold), we end up with something like this:

The above is the gold obtained from those computer motherboard pins and memory edges.
Note: If you use gold jewelry or any kind of gold/silver mixture, then chances are good that the blue liquid that you've been diluting/pouring off will contain reasonable amounts of dissolved silver. In that case, the silver can also be recovered by using the method I described in my earlier Dummies Guide to EASY silver bullion refining at home as a long term precious metal investment thread.
Having successfully removed almost all of the contaminants leaving the gold in an "almost" pure state, we're now going to process the gold one last time to remove any minute stubborn contaminants and end up with essentially 99.9% pure 24k gold ... woohoo !!
In this final step, we're going to do some "alchemy" ... just like the Ancients used to do !
Continued next post ...
Continued from previous post ...
One of the marvelous properties of gold, and what makes it so sought after and desirable, is that its a metal that that will never tarnish or rust or corrode.
Thats because gold is an extremely nonreactive metal. And because its so nonreactive and stable, non of the common acids will touch it ... not nitric, not hydrochloric, not sulphuric, etc.
So how DO we get the gold to dissolve ?
We'll take advantage of the Ancients knowledge of applied alchemy when they somehow discovered that a mixture of 1 part nitric and 3 parts hydrochloric performed the seemingly impossible ... it dissolved gold !
Because this combination of acids had the "power" to dissolve gold, which they considered to be the most "regal or noble" of metals, they gave it the special name of "Royal Water" ... or as they called it in Latin ... "Aqua Regia".
So, transfer the "washed" gold into one of the glass tubes and add a 1:3 mix of nitric and hydrochloric acids.
With the quantity of gold in this tutorial, using the plastic pipette, I added 5 drops of nitric and 15 drops of hydrochloric making a 1:3 mixture of Aqua Regia.
Immediately the gold starts to dissolve and the liquid starts to bubble and turn a nice yellow colour. This is a good sign that almost all contaminants have been removed previously. If the liquid has a blue or green tinge, then that means some copper must still be present with the gold.
Again, to speed up the reaction, we'll place the glass tube into a bath of boiling water.
Allow the reaction to continue until all the gold has dissolved. If the reaction stops and some gold still remains, then add a further small quantity of "Aqua Regia".

Ok, all the gold has dissolved leaving a yellow liquid behind ... for all intents and purposes, this yellow liquid is "liquid gold" ... so be very careful and DON'T spill any !
This yellow liquid may or not look slightly cloudy but either way, it won't do any harm to filter it.
So create a filter as shown here:

We're going to pour the yellow liquid through the filter and into a clean, empty glass tube.
Now I can't stress this part enough ... make sure that you have scrubbed and washed this glass tube until it's absolutely squeaky clean. The reason being that shortly, we'll be causing the dissolved gold to be "dropped" out of solution as a dark brown material that will settle on the bottom of the jar.
If the jar is NOT squeaky clean, some of the gold, as it drops out of solution, could stick to the sides of the glass jar giving it a very fine gold coating, similar to the fine silver coating on the back of a mirror. Then you'll waste time and effort in scraping this layer off to recover the gold in it.

After all the yellow liquid has been poured into and passed through the filter, using the water spray bottle, spray the inside of the filter to ensure that every last bit of yellow liquid containing the gold has been washed through the filter and into the glass bottle.
Ok, now dilute the yellow liquid with an equal volume of water.
We should be looking at something like the following:

We're now almost ready to precipitate or "drop" the gold back out of the yellow liquid but just before we do that, we need to completely neutralize any remaining traces of nitric acid that may still remain in the yellow liquid. The reason for this is simply that if we start to "drop" the gold in the form of a brown sediment, as soon as any of it appears, it will be immediately attacked by the remaining nitric/hydrochloric acid in the yellow solution and dissolved once again. So to prevent the "gold sediment" from going back into solution, where we don't want it to go, a simple way to prevent this is to first use urea to neutralize the nitric ... then successfully "drop" the sediment where it will eventually settle at the bottom of the glass jar.

I recommend using just a pinch of urea at a time. When added to the yellow liquid, you should notice bubbles being created by the urea. This is a sign that nitric acid is being neutralized. As the urea is used up, continue adding smaller and smaller amounts until there is no more bubbling to be seen ... at this point, the remaining nitric acid has been completely neutralized and we can proceed with "dropping" the gold back out of solution as a brown sediment.

Continued next post ...
One of the marvelous properties of gold, and what makes it so sought after and desirable, is that its a metal that that will never tarnish or rust or corrode.
Thats because gold is an extremely nonreactive metal. And because its so nonreactive and stable, non of the common acids will touch it ... not nitric, not hydrochloric, not sulphuric, etc.
So how DO we get the gold to dissolve ?
We'll take advantage of the Ancients knowledge of applied alchemy when they somehow discovered that a mixture of 1 part nitric and 3 parts hydrochloric performed the seemingly impossible ... it dissolved gold !
Because this combination of acids had the "power" to dissolve gold, which they considered to be the most "regal or noble" of metals, they gave it the special name of "Royal Water" ... or as they called it in Latin ... "Aqua Regia".
So, transfer the "washed" gold into one of the glass tubes and add a 1:3 mix of nitric and hydrochloric acids.
With the quantity of gold in this tutorial, using the plastic pipette, I added 5 drops of nitric and 15 drops of hydrochloric making a 1:3 mixture of Aqua Regia.
Immediately the gold starts to dissolve and the liquid starts to bubble and turn a nice yellow colour. This is a good sign that almost all contaminants have been removed previously. If the liquid has a blue or green tinge, then that means some copper must still be present with the gold.
Again, to speed up the reaction, we'll place the glass tube into a bath of boiling water.
Allow the reaction to continue until all the gold has dissolved. If the reaction stops and some gold still remains, then add a further small quantity of "Aqua Regia".

Ok, all the gold has dissolved leaving a yellow liquid behind ... for all intents and purposes, this yellow liquid is "liquid gold" ... so be very careful and DON'T spill any !
This yellow liquid may or not look slightly cloudy but either way, it won't do any harm to filter it.
So create a filter as shown here:

We're going to pour the yellow liquid through the filter and into a clean, empty glass tube.
Now I can't stress this part enough ... make sure that you have scrubbed and washed this glass tube until it's absolutely squeaky clean. The reason being that shortly, we'll be causing the dissolved gold to be "dropped" out of solution as a dark brown material that will settle on the bottom of the jar.
If the jar is NOT squeaky clean, some of the gold, as it drops out of solution, could stick to the sides of the glass jar giving it a very fine gold coating, similar to the fine silver coating on the back of a mirror. Then you'll waste time and effort in scraping this layer off to recover the gold in it.

After all the yellow liquid has been poured into and passed through the filter, using the water spray bottle, spray the inside of the filter to ensure that every last bit of yellow liquid containing the gold has been washed through the filter and into the glass bottle.
Ok, now dilute the yellow liquid with an equal volume of water.
We should be looking at something like the following:

We're now almost ready to precipitate or "drop" the gold back out of the yellow liquid but just before we do that, we need to completely neutralize any remaining traces of nitric acid that may still remain in the yellow liquid. The reason for this is simply that if we start to "drop" the gold in the form of a brown sediment, as soon as any of it appears, it will be immediately attacked by the remaining nitric/hydrochloric acid in the yellow solution and dissolved once again. So to prevent the "gold sediment" from going back into solution, where we don't want it to go, a simple way to prevent this is to first use urea to neutralize the nitric ... then successfully "drop" the sediment where it will eventually settle at the bottom of the glass jar.

I recommend using just a pinch of urea at a time. When added to the yellow liquid, you should notice bubbles being created by the urea. This is a sign that nitric acid is being neutralized. As the urea is used up, continue adding smaller and smaller amounts until there is no more bubbling to be seen ... at this point, the remaining nitric acid has been completely neutralized and we can proceed with "dropping" the gold back out of solution as a brown sediment.

Continued next post ...
Continued from previous post ...
Now that we've finally got our virtually pure "raw gold powder", we're going to make a gold "button"
Normally when melting precious metals such as silver and gold, a proper crucible made of heat resistant material is used. Such a material is designed to withstand the approximate 1000C temperature required to melt silver and gold.
However, we're going to go low tech in our approach and instead of a high temp crucible, we're going to use a potato placed on top of a brick ... yep, you read that correctly .. we're going to use a potato upon which we're going to melt our brown powder back into gold metal ! Believe it or not, but a slice of potato can easily and for long enough, withstand the sort of temperatures we need to melt gold..

A slice of potato is all thats needed and we need to slightly carbonize (burn) the surface. The carbon helps pick up any minuscule impurities remaining in the gold and also the carbon layer helps prevent the gold from adhering to the potato.

Now, we take a small quantity of our brown gold powder and place it on the potato surface. Because the brown powder is fine, we'll start with a low flame to prevent the pressure of the flame scattering (and losing !) our hard work. Once the brown powder has started to combine together, the flame is turned up high and melting begins. We do a small quantity at a time as it takes less time to melt and once melted, we continue adding additional small quantities and re-melt. This continues until all the brown powder has been used.

Eventually all the brown powder has been melted down.

And we're left holding a "button" of 99.9% pure gold to be added with the rest of our "long term investment" portfolio of gold "buttons". This example weighs approximately 1.5 gms and worth around $US86 at current market rates.

The above may have been long winded in explanation but I wanted to make sure that every step of the process was as clear as possible making it easy for anyone interested enough to have a go at themselves.
Summarized, the process is simply:
1. place the scrap gold into nitric acid to dissolve all other metal "impurities"
2. filter the resulting blue solution and trap the gold
3. apply a mixture of 1:3 nitric/hydrochloric acid (aqua regia) to the gold to completely dissolve it
4. filter the resulting yellow liquid to remove any remaining impurities
5. neutralize any left over nitric acid still in the yellow liquid with urea
6. use SMB to precipitate the brown coloured "gold sediment"
7. wash the sediment then leave until dried to a brown powder
7. melt the brown powder back into metallic gold
Good luck to those of you thinking of giving it a go !
Now that we've finally got our virtually pure "raw gold powder", we're going to make a gold "button"
Normally when melting precious metals such as silver and gold, a proper crucible made of heat resistant material is used. Such a material is designed to withstand the approximate 1000C temperature required to melt silver and gold.
However, we're going to go low tech in our approach and instead of a high temp crucible, we're going to use a potato placed on top of a brick ... yep, you read that correctly .. we're going to use a potato upon which we're going to melt our brown powder back into gold metal ! Believe it or not, but a slice of potato can easily and for long enough, withstand the sort of temperatures we need to melt gold..

A slice of potato is all thats needed and we need to slightly carbonize (burn) the surface. The carbon helps pick up any minuscule impurities remaining in the gold and also the carbon layer helps prevent the gold from adhering to the potato.

Now, we take a small quantity of our brown gold powder and place it on the potato surface. Because the brown powder is fine, we'll start with a low flame to prevent the pressure of the flame scattering (and losing !) our hard work. Once the brown powder has started to combine together, the flame is turned up high and melting begins. We do a small quantity at a time as it takes less time to melt and once melted, we continue adding additional small quantities and re-melt. This continues until all the brown powder has been used.

Eventually all the brown powder has been melted down.

And we're left holding a "button" of 99.9% pure gold to be added with the rest of our "long term investment" portfolio of gold "buttons". This example weighs approximately 1.5 gms and worth around $US86 at current market rates.

The above may have been long winded in explanation but I wanted to make sure that every step of the process was as clear as possible making it easy for anyone interested enough to have a go at themselves.
Summarized, the process is simply:
1. place the scrap gold into nitric acid to dissolve all other metal "impurities"
2. filter the resulting blue solution and trap the gold
3. apply a mixture of 1:3 nitric/hydrochloric acid (aqua regia) to the gold to completely dissolve it
4. filter the resulting yellow liquid to remove any remaining impurities
5. neutralize any left over nitric acid still in the yellow liquid with urea
6. use SMB to precipitate the brown coloured "gold sediment"
7. wash the sediment then leave until dried to a brown powder
7. melt the brown powder back into metallic gold
Good luck to those of you thinking of giving it a go !
Im so glad you decided to do a thread on processing gold as well... I read the silver one and really loved it, and maybe someday I will actually put
the knowledge together and do it myself...
I had always thought of trying to process junk computer/cell phones for precious metals but never had any clue how to go about it... but now I have a nice idea on where to start... Thanks a bunch
I had always thought of trying to process junk computer/cell phones for precious metals but never had any clue how to go about it... but now I have a nice idea on where to start... Thanks a bunch
Your posts are the most valuable I've seen on ATS in a long time. Thank you very much.
Once again nice thread and i will be trying this out after ive done the silver refining
I really enjoyed your thread on refining and recovering Silver, and now one on Gold, how lucky are we? (Answer: Very Lucky).
Thanks so much for a very concise and well thought out thread and presentation. I hope other Members will understand that a thread such as this is so much more valuable then simply grabbing a YouTube video and asking "What do you think?"
Bravo!
Johnny
Thanks so much for a very concise and well thought out thread and presentation. I hope other Members will understand that a thread such as this is so much more valuable then simply grabbing a YouTube video and asking "What do you think?"
Bravo!
Johnny
Excellent. Once again you have provided extremely useful knowledge in a simple paint by numbers fashion. Absolutely commendable. Thank you.
Your directions are spot on....i have one question though....
How many computers did it take to make the button?
I have 10 ripped apart, and the chips saved...also the mother boards etc....
I understand there is more to recovering gold from these parts but there is also platinum and even rhodium ive been told...
are these worth trying to get out, and does the process differ much for these?
meanwhile ill be pulling the chip pins and trying your method....
thanks....
I have some cell phone parts as well....but all have a plastic coating on them....
How many computers did it take to make the button?
I have 10 ripped apart, and the chips saved...also the mother boards etc....
I understand there is more to recovering gold from these parts but there is also platinum and even rhodium ive been told...
are these worth trying to get out, and does the process differ much for these?
meanwhile ill be pulling the chip pins and trying your method....
thanks....
I have some cell phone parts as well....but all have a plastic coating on them....
reply to post by tauristercus
I really enjoyed your thread on silver refining and I didn't know it was as simple as gold.
I have an easier process for refining gold, but I'm not allowed to share it publicly. Pm me if you'd like me to explain it to you.
I really enjoyed your thread on silver refining and I didn't know it was as simple as gold.
I have an easier process for refining gold, but I'm not allowed to share it publicly. Pm me if you'd like me to explain it to you.
Originally posted by stirling
Your directions are spot on....i have one question though....
How many computers did it take to make the button?
I have 10 ripped apart, and the chips saved...also the mother boards etc....
I understand there is more to recovering gold from these parts but there is also platinum and even rhodium ive been told...
are these worth trying to get out, and does the process differ much for these?
meanwhile ill be pulling the chip pins and trying your method....
thanks....
I have some cell phone parts as well....but all have a plastic coating on them....
Most of the gold in that tutorial button came from some small gold nuggets that I had purchased on eBay.
Additional small (and I emphasise, small) quantities of gold did come from the gold plated edge connectors of 2 memory sticks and gold plated pins removed from 2 old motherboards. You would need a sizable quantity of motherboard and memory sticks to end up with even a small amount of gold.
It's far more lucrative to get your hands on old, broken, unwanted, estate, etc gold items (rings, chains, etc) or gold nuggets from eBay and use them as your raw processing material.
Awesome and bookmarked again (after the silver guide)!
thanks OP . really amazing and useful info. Now you have convinced me on switching Hobbies.... my companies IT department has a whole room full of IT junk compiled over the years... time to give it a visit and offer to buy at employee rate
thanks OP . really amazing and useful info. Now you have convinced me on switching Hobbies.... my companies IT department has a whole room full of IT junk compiled over the years... time to give it a visit and offer to buy at employee rate
edit on 24-2-2012 by nagabonar because: (no reason given)
Superb, Ive always wanted to go panning for gold and may well do so, might even roll a few and wander upstream this weekend. I think the gold here is
amongst the purest in the world. I always wondered how I would get something other than flakes to sell.
reply to post by nagabonar
HAHA yeah I know, manager approval here to walk away with all sorts of old kit, its known as "trackable scrap"
I just wonder if there are any health and safety rules banning this yet in the UK, probably. Wont be long before us guys have to pee sitting down in case we miss and then slip lol
PS there are a lot of dangerous substances in old motherboard etc such as mercury and lithuim, cobalt and verious other nasties. Really dont go melting motherboards or anything folks unless you know exactly what you are doing.
HAHA yeah I know, manager approval here to walk away with all sorts of old kit, its known as "trackable scrap"
I just wonder if there are any health and safety rules banning this yet in the UK, probably. Wont be long before us guys have to pee sitting down in case we miss and then slip lol
PS there are a lot of dangerous substances in old motherboard etc such as mercury and lithuim, cobalt and verious other nasties. Really dont go melting motherboards or anything folks unless you know exactly what you are doing.
edit on 24-2-2012 by Maponos because: (no reason
given)
reply to post by tauristercus
That is really neat and I did not realize how simple it was to do it. Will have to start collecting scrap gold.
That is really neat and I did not realize how simple it was to do it. Will have to start collecting scrap gold.
reply to post by tauristercus
I would like to thank you, you have inspired me to start my own business in the scrap valuable metals trade, also as a joint business electrical scrap collector.
The Information you have given us is invaluable, and if people delve deeper into the world of valuable metals, Theres alot to uncover.
Thank you once again.
I would like to thank you, you have inspired me to start my own business in the scrap valuable metals trade, also as a joint business electrical scrap collector.
The Information you have given us is invaluable, and if people delve deeper into the world of valuable metals, Theres alot to uncover.
Thank you once again.
Here we go again..
I've been eagerly awaiting this thread..
Thanks so much..
I have begun collecting 'samples' and will continue to do so until I have enough to melt down...
It's incredible how many different items there are out there that contain or include some amount of gold or silver..
Keep your eyes open people and keep looking..
It's amazing just how few of those chip board connectors you need in order to get that amount of gold.
I think the main stream 'recyclers tend to use weights like 'a tonne' of scrap in order to stop the likes of you and me from delving into a simple extraction process so the money stays where they want it.. in their pockets..
ETA, as an example of one of these 'other' types of gold sources I was talking about.. take a look at this tea pot
www.ebay.co.uk...
I've been eagerly awaiting this thread..
Thanks so much..
I have begun collecting 'samples' and will continue to do so until I have enough to melt down...
It's incredible how many different items there are out there that contain or include some amount of gold or silver..
Keep your eyes open people and keep looking..
It's amazing just how few of those chip board connectors you need in order to get that amount of gold.
I think the main stream 'recyclers tend to use weights like 'a tonne' of scrap in order to stop the likes of you and me from delving into a simple extraction process so the money stays where they want it.. in their pockets..
ETA, as an example of one of these 'other' types of gold sources I was talking about.. take a look at this tea pot
www.ebay.co.uk...
edit on 24-2-2012 by Extralien because: (no reason given)
reply to post by tauristercus
Excellent tutorial. Very informative and clearly written.
But.......
Im not sure if I have missed something, but how do you "drop the gold sediment" out of the yellow liquid that has been neutralised by the urea?
How is the powder extracted from the liquid?
Do you dry the powder once it has been seperated?
Is that just a process of air drying?
Excellent tutorial. Very informative and clearly written.
But.......
Im not sure if I have missed something, but how do you "drop the gold sediment" out of the yellow liquid that has been neutralised by the urea?
How is the powder extracted from the liquid?
Do you dry the powder once it has been seperated?
Is that just a process of air drying?
very cool.
i'm wanting to start buying scrap gold from individuals, but how do i tell if its real/plated or fake?
is a chemical test better or an electronic tester?
how much does it cost you in materials to refine a certain amount of gold?
i'm wanting to start buying scrap gold from individuals, but how do i tell if its real/plated or fake?
is a chemical test better or an electronic tester?
how much does it cost you in materials to refine a certain amount of gold?
new topics
-
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media: 26 minutes ago -
Any one suspicious of fever promotions events, major investor Goldman Sachs card only.
The Gray Area: 2 hours ago -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology: 7 hours ago -
Electrical tricks for saving money
Education and Media: 10 hours ago -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 11 hours ago
top topics
-
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News: 11 hours ago, 9 flags -
Cats Used as Live Bait to Train Ferocious Pitbulls in Illegal NYC Dogfighting
Social Issues and Civil Unrest: 15 hours ago, 8 flags -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues: 12 hours ago, 4 flags -
Electrical tricks for saving money
Education and Media: 10 hours ago, 4 flags -
Sunak spinning the sickness figures
Other Current Events: 12 hours ago, 3 flags -
Late Night with the Devil - a really good unusual modern horror film.
Movies: 14 hours ago, 2 flags -
Any one suspicious of fever promotions events, major investor Goldman Sachs card only.
The Gray Area: 2 hours ago, 2 flags -
The Good News According to Jesus - Episode 1
Religion, Faith, And Theology: 17 hours ago, 1 flags -
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology: 7 hours ago, 0 flags -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media: 26 minutes ago, 0 flags
active topics
-
God's Righteousness is Greater than Our Wrath
Religion, Faith, And Theology • 25 • : andy06shake -
Russia Ukraine Update Thread - part 3
World War Three • 5730 • : Arbitrageur -
Nearly 70% Of Americans Want Talks To End War In Ukraine
Political Issues • 28 • : andy06shake -
VP's Secret Service agent brawls with other agents at Andrews
Mainstream News • 43 • : Hakaiju -
Everest-sized ‘Devil comet’ Pons-Brooks Visible Now
Space Exploration • 17 • : Compendium -
Nakedeye Mother of Dragons Comet Is Here!
Space Exploration • 5 • : Compendium -
HORRIBLE !! Russian Soldier Drinking Own Urine To Survive In Battle
World War Three • 38 • : BernnieJGato -
15 Unhealthiest Sodas On The Market
Health & Wellness • 43 • : JPRCrastney -
University of Texas Instantly Shuts Down Anti Israel Protests
Education and Media • 0 • : FlyersFan -
Sunak spinning the sickness figures
Other Current Events • 9 • : Ohanka
