It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: cooperton
originally posted by: Phantom423
www.abovetopsecret.com...
www.abovetopsecret.com...
It's all in the links I posted. I suggest you read them.
There is nothing in there about peptide monomers self-assembling into polypeptides. If I missed it, please quote it.
What is a peptide monomer?
a reply to: Phantom423
Conformational features of the Aβ42 peptide monomer and its interaction with the surrounding solvent
LINK -
pubs.rsc.org...
Conformational features of the Aβ42 peptide monomer and its interaction with the surrounding solvent
LINK -
pubs.rsc.org...
originally posted by: cooperton
originally posted by: Phantom423
www.abovetopsecret.com...
www.abovetopsecret.com...
It's all in the links I posted. I suggest you read them.
There is nothing in there about peptide monomers self-assembling into polypeptides. If I missed it, please quote it.
Did you figure it out yet?
www.abovetopsecret.com...
originally posted by: Phantom423
What is a peptide monomer?
A peptide monomer is a single amino acid. Amino acids polymerize to form polypeptide chains. This process requires DNA code, transcription, translation, and often post-translational modification to be able to function. This process does not happen through spontaneous self-assembly, especially to the degree of making functional length proteins.
originally posted by: cooperton
originally posted by: Phantom423
What is a peptide monomer?
A peptide monomer is a single amino acid. Amino acids polymerize to form polypeptide chains. This process requires DNA code, transcription, translation, and often post-translational modification to be able to function. This process does not happen through spontaneous self-assembly, especially to the degree of making functional length proteins.
Mostly correct. What's wrong is that you forgot about PROCESS. The process is self assembling. No one ever said that you throw 2 gm lysine, 4 gm adenine etc into a pot of water and out pops a protein (although in some cases, that can happen).
And it is spontaneous. If it were not spontaneous, no cell regeneration would happen. The rate of cell turnover every day is evidence of that.
It's the PROCESS that is self assembling. There is no third party involved - no guy in the sky, no Super Mario brothers, no men in black.
edit on 28-7-2019 by Phantom423 because: (no reason given)
originally posted by: Phantom423
(peptide bonds are) is spontaneous. If it were not spontaneous, no cell regeneration would happen. The rate of cell turnover every day is evidence of that.
That's textbook incorrect. Peptide bonds require energy input (ATP) and are non-spontaneous reactions:
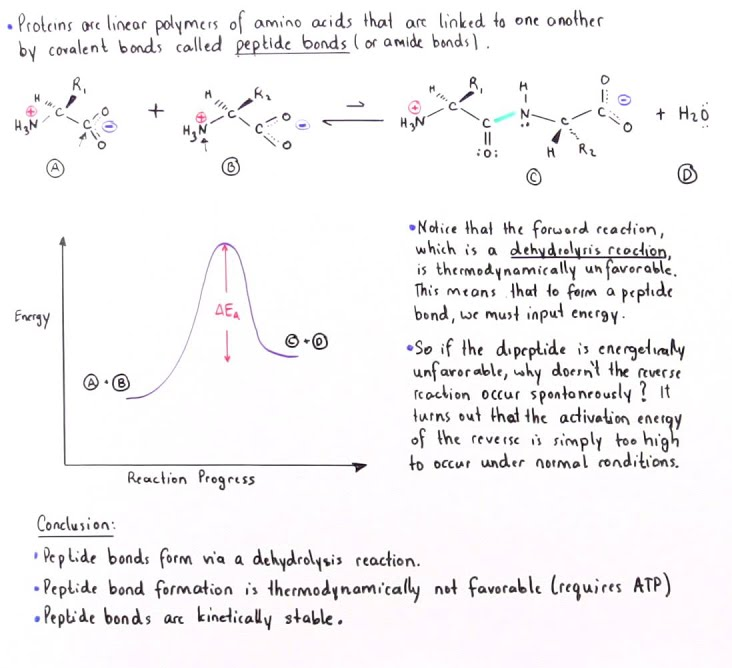
This is why the process of translation (amino acid polymerization) requires energy. Without this process, amino acids cannot self-assemble (polymerize on their own).
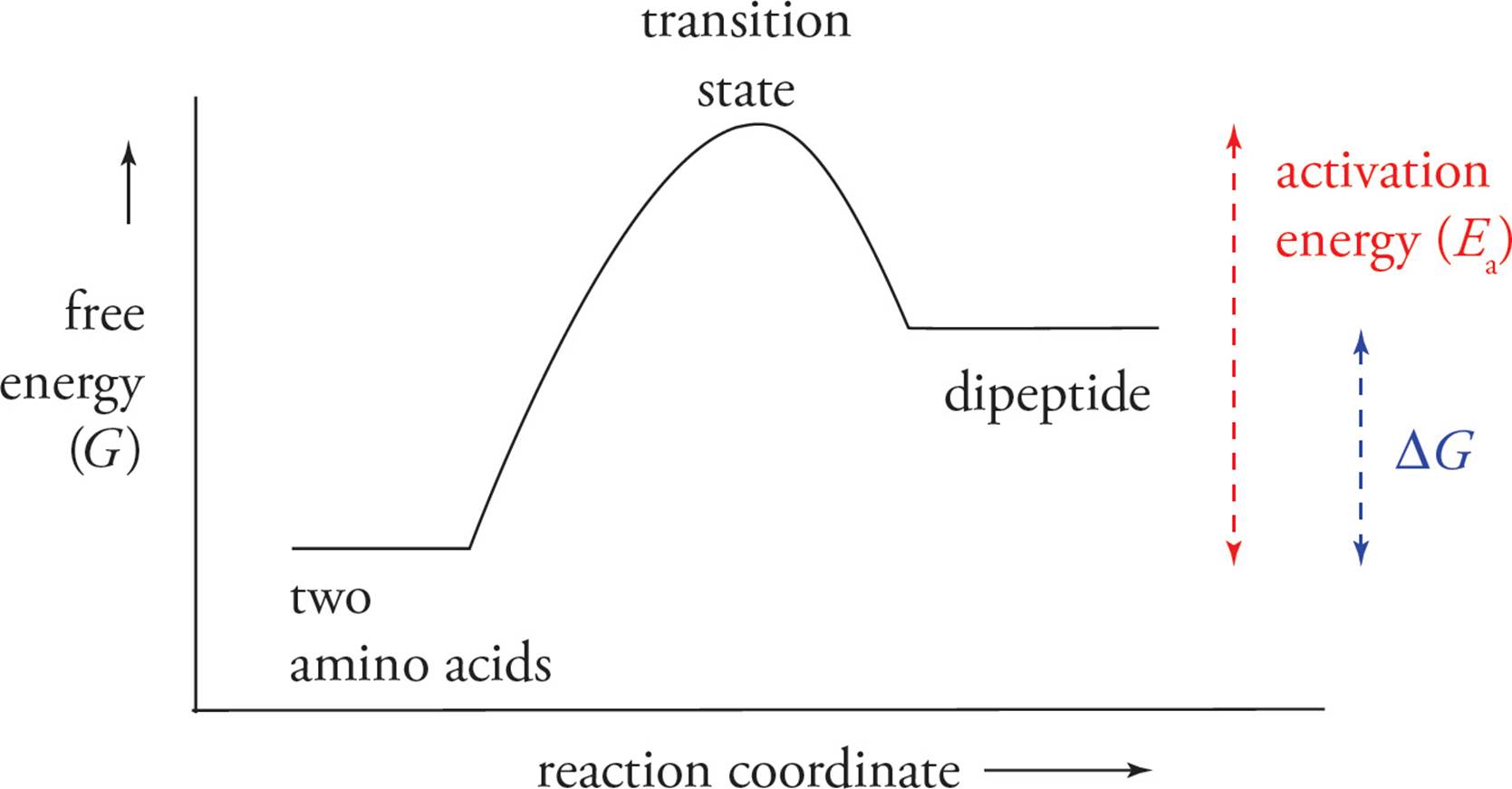
To make a peptide bond the tRNA must assemble enough energy in the form of ATP to surpass the activation energy. Because amino acid polymerization is a non-spontaneous reaction, the assemblage of all the proteins required for a muscle unit is irreducibly complex and could not have come to be by step-by-step mutations or random spontaneous formations.
edit on 28-7-2019 by cooperton because: (no reason given)
I am having a blast watching other science seriously challenge an ideology most people just accepted when their public school system taught it to them
as a fact of life. They and the school system never dug deeply into it like is being done in this thread. It makes a critical thinking person without
bias re-evaluate the whole thing.
a reply to: cooperton
The energy is inherent to the system. It's a thermodynamic process.
Your example is not correct. Peptide bond formation CONSUMES energy. The energy is provided by ATP.
The peptide bond itself is a condensation reaction. All chemical bonds - whether they are broken or formed - require energy. And it is spontaneous because ATP, as part of the process, is available as the energy donor. No magic is required.
You seem to leave out PROCESS for some illogical reason. Process means that all components of a system are available for a particular reaction - break up or break down. Leave out a component and the system doesn't work. I don't know what's so difficult about the concept. The universe is built on process and systems.
Also that model you posted comes from AK Lectures - which is wrong - it doesn't describe the thermodynamics properly. The guy has been called out on it several times.
The energy is inherent to the system. It's a thermodynamic process.
Your example is not correct. Peptide bond formation CONSUMES energy. The energy is provided by ATP.
The peptide bond itself is a condensation reaction. All chemical bonds - whether they are broken or formed - require energy. And it is spontaneous because ATP, as part of the process, is available as the energy donor. No magic is required.
You seem to leave out PROCESS for some illogical reason. Process means that all components of a system are available for a particular reaction - break up or break down. Leave out a component and the system doesn't work. I don't know what's so difficult about the concept. The universe is built on process and systems.
Also that model you posted comes from AK Lectures - which is wrong - it doesn't describe the thermodynamics properly. The guy has been called out on it several times.
edit on 28-7-2019 by Phantom423 because: (no reason given)
edit on 28-7-2019 by Phantom423 because: (no reason
given)
edit on 28-7-2019 by Phantom423 because: (no reason given)
a reply to: cooperton
And once again, I'll post this paper which describes the thermodynamics of peptide bond formation:
Peptide self-assembly: thermodynamics
and kinetics
Juan Wang,a Kai Liu,ab Ruirui Xinga and Xuehai Yan*ac
shimineh.com...
Perhaps you should read it in detail. You obviously do not understand how thermodynamics works in living systems.
And once again, I'll post this paper which describes the thermodynamics of peptide bond formation:
Peptide self-assembly: thermodynamics
and kinetics
Juan Wang,a Kai Liu,ab Ruirui Xinga and Xuehai Yan*ac
shimineh.com...
Self-assembling systems play a significant role in physiological functions and have therefore attracted tremendous attention due to their great potential for applications in energy, biomedicine and nanotechnology. Peptides, consisting of amino acids, are among the most popular building blocks and programmable molecular motifs. Nanostructures and materials assembled using peptides exhibit important potential for green-life new technology and biomedical applications mostly because of their bio-friendliness and reversibility. The formation of these ordered nanostructures pertains to the synergistic effect of various intermolecular non-covalent interactions, including hydrogen-bonding, p–p stacking, electrostatic, hydrophobic, and van der Waals interactions. Therefore, the self-assembly process is mainly driven by thermodynamics; however, kinetics is also a critical factor in structural modulation and function integration. In this review, we focus on the influence of thermodynamic and kinetic factors on structural assembly and regulation based on different types of peptide building blocks, including aromatic dipeptides, amphiphilic peptides, polypeptides, and amyloid-relevant peptides.
Perhaps you should read it in detail. You obviously do not understand how thermodynamics works in living systems.
edit on 28-7-2019 by Phantom423 because: (no reason given)
a reply to: cooperton
You need to get a good physical chemistry book and start from scratch. Nothing happens in a vacuum. You're picking and choosing again to make a point which has been invalidated about a thousand times.
Seriously, you don't understand physical chemistry - or any chemistry for that matter. Get a few good books and study.
You need to get a good physical chemistry book and start from scratch. Nothing happens in a vacuum. You're picking and choosing again to make a point which has been invalidated about a thousand times.
Seriously, you don't understand physical chemistry - or any chemistry for that matter. Get a few good books and study.
originally posted by: cooperton
originally posted by: Phantom423
www.abovetopsecret.com...
www.abovetopsecret.com...
It's all in the links I posted. I suggest you read them.
There is nothing in there about peptide monomers self-assembling into polypeptides. If I missed it, please quote it.
This guy is a catch phrase warrior. If your paper doesn't contain that exact phrase, it's automatically all wrong and can't happen.
originally posted by: Blue_Jay33
I am having a blast watching other science seriously challenge an ideology most people just accepted when their public school system taught it to them as a fact of life. They and the school system never dug deeply into it like is being done in this thread. It makes a critical thinking person without bias re-evaluate the whole thing.
How, by Coop repeatedly proving over and over that he doesn't grasp the basics of any of it?
edit on 7 28 19 by Barcs because: (no reason
given)
originally posted by: Phantom423
a reply to: cooperton
All chemical bonds - whether they are broken or formed - require energy.
Again, that is textbook not true. Some chemical reactions are spontaneous because their Gibb's free energy quotient is negative, meaning the reactants have more energy than the products (and they are therefore releasing energy).
And it is spontaneous because ATP, as part of the process, is available as the energy donor.
No, it is textbook non-spontaneous because it requires ATP. If the reaction were spontaneous, it would not require external energy sources to occur. Spontaneity of a reaction is measured by Gibbs free energy equation:
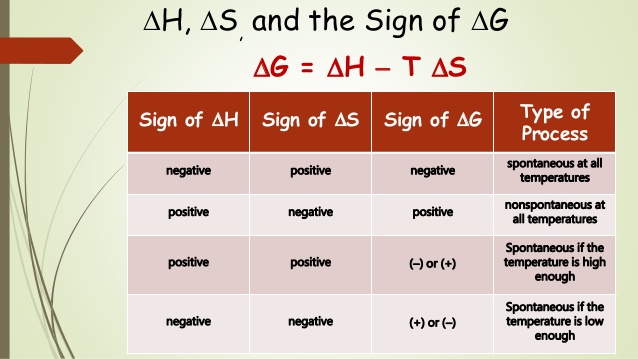
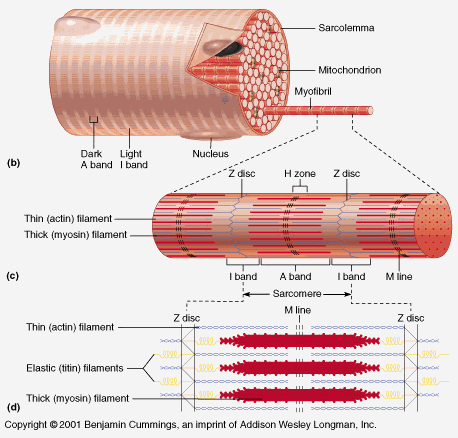
amino acid polymerization is a non-spontaneous reaction. This is why it requires tRNA to facilitate the polymerization of amino acids into functional protein sequences, this also requires the mRNA sequence that codes for the sequence of the protein. This does not happen without this machinery, or the proper DNA code that has the correct sequence to pass on to the mRNA.
For these reasons, creating all the necessary proteins for the muscle unit could not have occurred by sequential mutations because all protein components must be in place. And because amino acid polymerization doesn't occur spontaneously, it makes such a hurdle even more impossible. Not to mention the titin protein has a DNA sequence of over 100,000 base pairs. It is absurd to think this could have come to be through random mutations, especially considering it would still need all the other proteins involved in the muscle unit to come to be in the same way!
originally posted by: Phantom423
a reply to: cooperton
And once again, I'll post this paper which describes the thermodynamics of peptide bond formation
You need to understand this distinction:
1) A peptide bond is the polymerization of amino acids.
2) The "peptide self-assembly" paper that you keep sending is about the amalgamation of tertiary proteins, meaning the folding of polypeptides into a tertiary structure. It is totally different than amino acid polymerization.
originally posted by: Barcs
How, by Coop repeatedly proving over and over that he doesn't grasp the basics of any of it?
Which aspect of biology did I misrepresent? Where am I wrong? Or maybe you just don't understand biology?
edit on 28-7-2019 by cooperton
because: (no reason given)
"Protein Complement System" in the human body is incredibly complex.
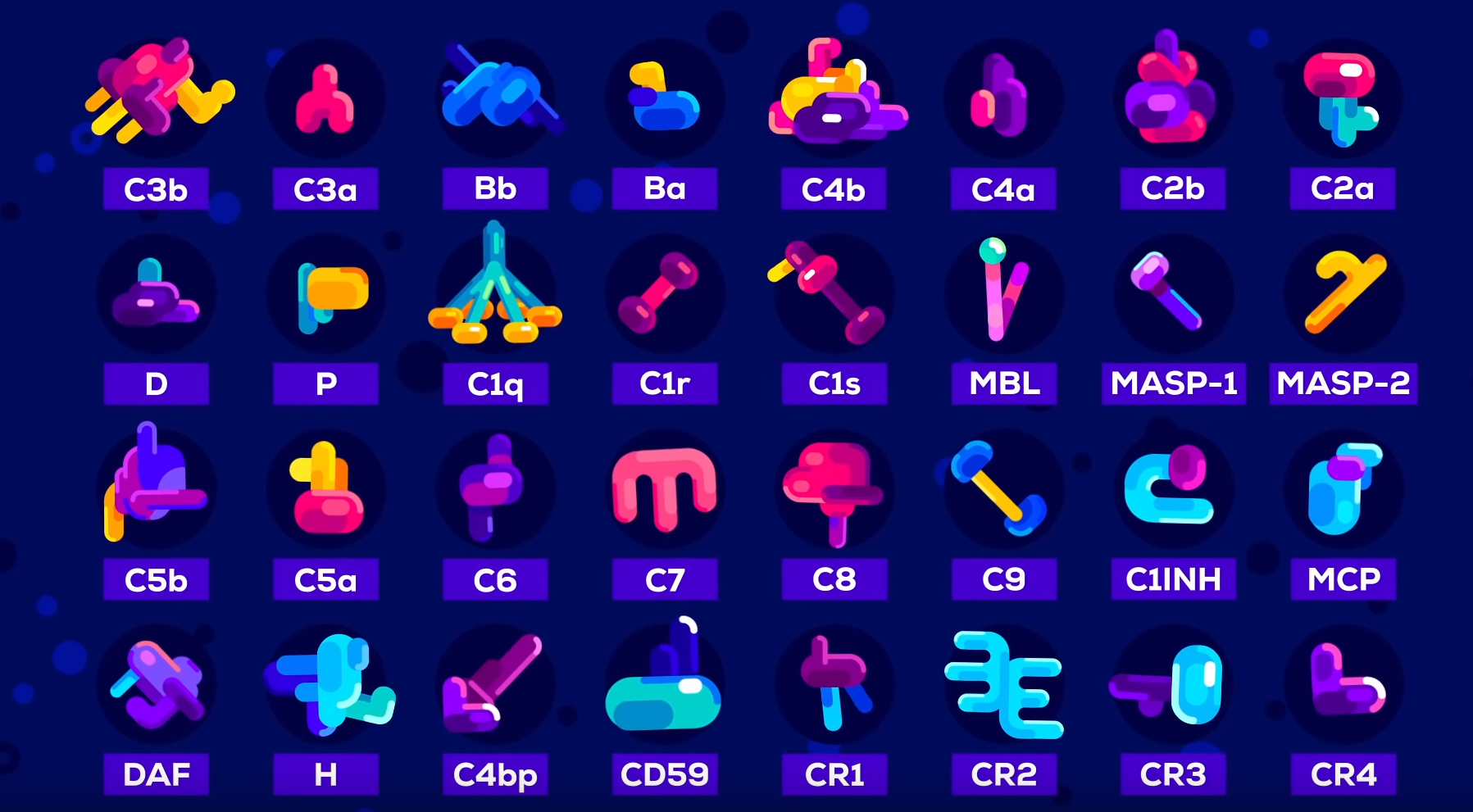
Both these posters saying you don't understand biology and chemistry, don't know everything about it either.
Meanwhile cooperton is posting credible pictures, diagrams and quotes and they are just saying sorry that is just wrong....I guess it's up to the readers of this thread to decide if what he is posting is 100% wrong.
Or are they just saying that about the posted science because of such a staunch bias ?

Both these posters saying you don't understand biology and chemistry, don't know everything about it either.
Meanwhile cooperton is posting credible pictures, diagrams and quotes and they are just saying sorry that is just wrong....I guess it's up to the readers of this thread to decide if what he is posting is 100% wrong.
Or are they just saying that about the posted science because of such a staunch bias ?
edit on 28-7-2019 by Blue_Jay33 because: (no reason
given)
a reply to: cooperton
You are 100% wrong. Come back when you know something about physical chemistry and thermodynamics. Until then, it's a hopeless conversation. You're piecemealing things together like a jigsaw puzzle with no evidence.
You don't understand process. You don't understand systems. You don't understand self assembly.
I'm done with this. Please consult YouTube scientist to validate your position. I have no doubt that he can do that.
Bye.
You are 100% wrong. Come back when you know something about physical chemistry and thermodynamics. Until then, it's a hopeless conversation. You're piecemealing things together like a jigsaw puzzle with no evidence.
You don't understand process. You don't understand systems. You don't understand self assembly.
I'm done with this. Please consult YouTube scientist to validate your position. I have no doubt that he can do that.
Bye.
originally posted by: Phantom423
a reply to: cooperton
You are 100% wrong.
Explain where I am wrong and we can go from there. What science did I get wrong? Use actual examples, not libelous blanket statements like "you are 100% wrong". Or is this dodge you are trying to pull off your way of conceding to the fact that amino acids cannot self-polymerize?
originally posted by: cooperton
originally posted by: Phantom423
a reply to: cooperton
You are 100% wrong.
Explain where I am wrong and we can go from there. What science did I get wrong? Use actual examples, not libelous blanket statements like "you are 100% wrong". Or is this dodge you are trying to pull off your way of conceding to the fact that amino acids cannot self-polymerize?
You are 100% wrong because you're avoiding the process/system like the plague.
1. Self assembly of peptides/proteins is a SYSTEM and a PROCESS.
2. If any component is missing i.e. ATP, the SYSTEM fails
3. All chemical bonds require energy- IT DOESN'T MATTER WHAT'S BONDING. PEPTIDE BOND FORMATION REQUIRES ENERGY - JUST LIKE ANY OTHER CHEMICAL BOND.
4. The process of PEPTIDE BOND FORMATION AND PROTEINS REQUIRES ALL THE COMPONENTS OF THE SYSTEM TO BE PRESENT.
5. PEPTIDE BOND FORMATION AND PROTEINS ARE PART OF A SYSTEM.
6. THE SYSTEM IS SPONTANEOUS - no OUTSIDE INTERVENTION IS REQUIRED - GUY IN THE SKY, SUPER MARIO BROTHERS, MEN IN BLACK.
7. Tertiary and quaternary confirgurations ARE PART OF THE SYSTEM. IT DOESN'T MATTER WHAT TYPE OF BONDS THEY ARE FORMING - VAN DER WAALS, HYDROGEN, COVALENT BONDS, IONIC BONDS, POLAR, METALLIC - IT DOESN'T MATTER - THEY ALL REQUIRE ENERGY.
8. THE INITIAL AMINO ACID-AMINO ACID BOND REQUIRES ATP BECAUSE IT IS A CHEMICAL BOND - ALL CHEMICAL BONDS REQUIRE ENERGY. IT IS A CONDENSATION REACTION RELEASING H20. IT IS PART OF THE SYSTEM. THE SYSTEM IS SPONTANEOUS. AMINO ACID BONDING IS SPONTANEOUS IN THE SYSTEM BECAUSE THE SYSTEM REQUIRES ATP CATALYST AS THE CATALYST.
That is a description as to why you are 100% wrong.
Your ignorance is astounding.

edit on 29-7-2019 by Phantom423 because: (no reason given)
edit on 29-7-2019 by Phantom423 because: (no reason given)
originally posted by: Phantom423
2. If any component is missing i.e. ATP, the SYSTEM fails
Exactly. Without all the components for the muscle unit, the system fails. Which is why it is irreducibly complex.
6. THE SYSTEM IS SPONTANEOUS - no OUTSIDE INTERVENTION IS REQUIRED
What are you talking about? There are plenty of non-spontaneous reactions in the system of the human body.
7. Tertiary and quaternary confirgurations ARE PART OF THE SYSTEM. IT DOESN'T MATTER WHAT TYPE OF BONDS THEY ARE FORMING - VAN DER WAALS, HYDROGEN, COVALENT BONDS, IONIC BONDS, POLAR, METALLIC - IT DOESN'T MATTER - THEY ALL REQUIRE ENERGY.
8. THE INITIAL AMINO ACID-AMINO ACID BOND REQUIRES ATP BECAUSE IT IS A CHEMICAL BOND - ALL CHEMICAL BONDS REQUIRE ENERGY. IT IS A CONDENSATION REACTION RELEASING H20. IT IS PART OF THE SYSTEM. THE SYSTEM IS SPONTANEOUS. AMINO ACID BONDING IS SPONTANEOUS IN THE SYSTEM BECAUSE THE SYSTEM REQUIRES ATP CATALYST AS THE CATALYST.
undo caps locks
Yes and peptide bonds are by definition non-spontaneous, because they require ATP. I don't get what you don't get about that. Spontaneous reactions don't require external energy. Non-spontaneous reactions do. A Peptide bond is a dehydration synthesis reaction:
"A dehydration synthesis is an endergonic (or 'energy in') type of reaction that cannot take place without the input of energy from somewhere else. It is non-spontaneous, and by the second law of thermodynamics will not take place on its own."
source
a reply to: cooperton
The funny thing is they keep saying you are wrong, but fail to realize they are...
while the examples they provide also say they are wrong...
It’s also funny they introduced thermodynamics because the laws state supernatural means would have been required
To create the Universe...
The funny thing is they keep saying you are wrong, but fail to realize they are...
while the examples they provide also say they are wrong...
It’s also funny they introduced thermodynamics because the laws state supernatural means would have been required
To create the Universe...
new topics
-
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies: 3 minutes ago -
Hurt my hip; should I go see a Doctor
General Chit Chat: 52 minutes ago -
Israel attacking Iran again.
Middle East Issues: 2 hours ago -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 2 hours ago -
When an Angel gets his or her wings
Religion, Faith, And Theology: 2 hours ago -
Comparing the theology of Paul and Hebrews
Religion, Faith, And Theology: 3 hours ago -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 4 hours ago -
Boston Dynamics say Farewell to Atlas
Science & Technology: 4 hours ago -
I hate dreaming
Rant: 5 hours ago -
Man sets himself on fire outside Donald Trump trial
Mainstream News: 7 hours ago
top topics
-
The Democrats Take Control the House - Look what happened while you were sleeping
US Political Madness: 8 hours ago, 18 flags -
In an Historic First, In N Out Burger Permanently Closes a Location
Mainstream News: 10 hours ago, 16 flags -
A man of the people
Medical Issues & Conspiracies: 15 hours ago, 11 flags -
Biden says little kids flip him the bird all the time.
Politicians & People: 7 hours ago, 8 flags -
Man sets himself on fire outside Donald Trump trial
Mainstream News: 7 hours ago, 7 flags -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs: 4 hours ago, 6 flags -
Israel attacking Iran again.
Middle East Issues: 2 hours ago, 5 flags -
Michigan school district cancels lesson on gender identity and pronouns after backlash
Education and Media: 2 hours ago, 4 flags -
4 plans of US elites to defeat Russia
New World Order: 17 hours ago, 4 flags -
Boston Dynamics say Farewell to Atlas
Science & Technology: 4 hours ago, 4 flags
active topics
-
Thousands Of Young Ukrainian Men Trying To Flee The Country To Avoid Conscription And The War
Other Current Events • 53 • : ghandalf -
WF Killer Patents & Secret Science Vol. 1 | Free Energy & Anti-Gravity Cover-Ups
General Conspiracies • 0 • : 727Sky -
Boston Dynamics say Farewell to Atlas
Science & Technology • 5 • : Caver78 -
Hurt my hip; should I go see a Doctor
General Chit Chat • 7 • : randomtangentsrme -
Biden says little kids flip him the bird all the time.
Politicians & People • 16 • : stelth2 -
When an Angel gets his or her wings
Religion, Faith, And Theology • 2 • : stelth2 -
MULTIPLE SKYMASTER MESSAGES GOING OUT
World War Three • 51 • : Zaphod58 -
Israel attacking Iran again.
Middle East Issues • 19 • : stelth2 -
Pentagon acknowledges secret UFO project, the Kona Blue program | Vargas Reports
Aliens and UFOs • 7 • : Ophiuchus1 -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 549 • : cherokeetroy
