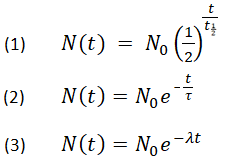It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
originally posted by: cooperton
originally posted by: edmc^2
Problem with this concept (6 literal days) is you have to come up with an explanation like yours to make it feasible while reality shows otherwise.
What evidence is there that the earth is very old?
Sedimentation occurs very rapidly, as demonstrated by polystrate fossils which are trees that persisted through multiple deposition events (poly- "many"; strate - "layers".
Seriously, there is no empirical evidence to demonstrate that sedimentation takes lots of time:
It is a rapid process. Don't fall for the junk science, look for the empirical evidence.
coop,
I'm using science - radiometric dating (not c14 dating - which I know is the wrong tool for dating inorganic materials).
Empirical evidence points to an older earth - according to the current data.
Example - the decay of Uranium isotopes.
scienceline.ucsb.edu...
BTW - the reason why c14-dating method can't be used for dating fossils is the fact the half-life of carbon14 is around 10,000 down to 6000. Hence, the radiometric dating was chosen.
But by choosing this method, archeologist arrived wrong conclusions. Instead of fossils dating around 6000 to 10,000 (or even 50,000) years, they come up with billions of years.
As the OOPA (Out Of Place Artifacts) such as those posted above - testify to a massive flood not the age of the earth.
Then again, I might be wrong and you're correct.
originally posted by: Theocracy4America
a reply to: edmc^2
I hope not via the debunked carbon dating methods.
Theo,
I studied both methodologies - Radiometric and C14 dating.
Each tool has a specific use. One is for inorganic materials (such as rock) and the other for organic (artifacts/bones).
Problem is, evolutionists, borrowed the former to prove their claim of fossils being millions of years old.
It's like trying t dig a very delicate flower with a backhoe - wrong tool.
a reply to: edmc^2
C14 has little to do with the age of the Earth, but it does make a nice strawman for those who don't care about facts.
However, big news on the C14 calibration front.
phys.org...
C14 has little to do with the age of the Earth, but it does make a nice strawman for those who don't care about facts.
However, big news on the C14 calibration front.
phys.org...
edit on 12/14/2018 by Phage because: (no reason given)
originally posted by: Phage
a reply to: edmc^2
C14 has little to do with the age of the Earth, but it does make a nice strawman for those who don't care about facts.
However, big news on the C14 calibration front.
phys.org...
In this new effort, the researchers report on the finding of two stalagmites in a Chinese cave that offer an accurate measure of such ratios going back approximately 54,000 years. The half-life of carbon-14 is 5,370 years. Read more at: phys.org...
54,000 years - is within what I said (i.e. 6000 to 50,000).
But still, the point is, since this methodology is the wrong tool for dating "fossilized" materials (as it disproves the claim of fossils being millions of years old) so they (evolution-archeologist) went to radiometric dating.
edit on 14-12-2018 by edmc^2 because: dd
originally posted by: Phage
a reply to: edmc^2
As I said, C14 dating makes a good strawman argument for those of limited knowledge (or with an agenda) when it comes to geologic timescales.
Which is the topic. No?
Yeah, I guess you can say that. C14 to prove a young earth while rejecting radioactive decays of uranium isotopes - n such. On the flip side, the other use radiometric dating to prove "fossils" being in the millions instead of thousands of years.
Topic though is that there's a third view which explains the currently accepted age of the earth.
Thanks for the link though - I'll add it to my collections.
edit on 14-12-2018 by edmc^2 because: bv
a reply to: edmc^2
It would seem that you are not using a "flip side" at all, but instead, the same strawman.
On the flip side, the other use radiometric dating to prove "fossils" being in the millions instead of thousands of years.
It would seem that you are not using a "flip side" at all, but instead, the same strawman.
edit on 12/14/2018 by Phage because: (no reason given)
originally posted by: edmc^2
originally posted by: Phage
a reply to: edmc^2
C14 has little to do with the age of the Earth, but it does make a nice strawman for those who don't care about facts.
However, big news on the C14 calibration front.
phys.org...
In this new effort, the researchers report on the finding of two stalagmites in a Chinese cave that offer an accurate measure of such ratios going back approximately 54,000 years. The half-life of carbon-14 is 5,370 years. Read more at: phys.org...
54,000 years - is within what I said (i.e. 6000 to 50,000).
But still, the point is, since this methodology is the wrong tool for dating "fossilized" materials (as it disproves the claim of fossils being millions of years old) so they (evolution-archeologist) went to radiometric dating.
I really hate to be pedantic here but 14C dating is a form of radio metric dating. Radiometric simply means using the known half-life of Radioactive elements and 14C is indeed a radioactive element. Thus, it too is a method of radio metric dating.
And just to take the pedantics a little farther, I’m not sure what an evolution-archaeologist is but archaeologists don’t study anything millions of years old. They study the physical remains of human culture and civilization. Typically that ends in the late Pleistocene/ early Holocene when humans began building megalithic monuments like Gobei Tepe, early PPNA sites, early cities like Jericho etc... so Archaeology only goes back about 12Ka or so.
If you’re talking fossilized remains of archaic hominids, then that’s studied by Anthropologistd. Ancient animal Fossils, Paleontologists. Dating of rock layers, Geologists.
So it’s not a matter of moving goal posts to another dating method, it’s a matter Of the proper people using the correct tools to study a find or site properly.
originally posted by: edmc^2
Example - the decay of Uranium isotopes.
scienceline.ucsb.edu...
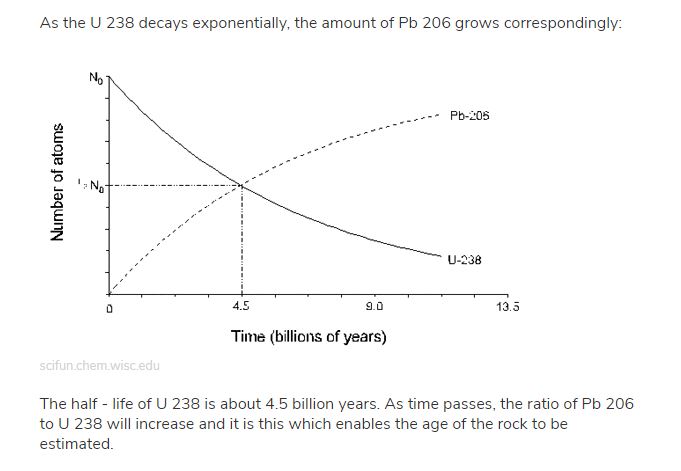
"A geologist can pick up a rock from a mountainside somewhere, and bring it back to the lab, and separate out the individual minerals that compose the rock. They can then look at a single mineral, and using an instrument called a mass spectrometer, they can measure the amount of parent and the amount of daughter in that mineral. The ratio of the parent to daughter then can be used to back-calculate the age of that rock."
The problem with this is that you cannot know the original ratio of the rock. There is no way to know the original uranium-lead ratio when the sample was formed. Decay rate is very well known, but without the original ratio, you're shooting in the dark.
For example, a 50%-50% Uranium-lead sample is found. We could assume - key word being assume - that the original sample was 100% Uranium, indicating a 4.46 billion year old sample, but we have no way of knowing the original ratios. For all we know the concentration was originally 50.00001%-49.99999% Uranium-lead, which would make the sample much, much, much younger. There is no way around this assumption.
edit on 15-12-2018 by cooperton because: (no reason given)
a reply to: cooperton
You are absolutely 100% wrong that you need the original ratio to calculate the age. This is another example of your ignorance of science and mathematics:
In chemical kinetics the slowest step in any reaction is the rate determining step. The conversion of uranium 238 to lead 206 is a FIRST ORDER PROCESS which can be represented by a first order differential equation. The reaction is dependent on only ONE component in the reaction. There is no requirement for a previous ratio or value because the reaction is LINEAR.



You and the idiots at the Creation Institute have used that excuse ad infinitum to prove that the age of the Earth or rocks or whatever cannot be known.
So now it's your turn - present your mathematical proof that the original isotopic ratio is required to determine the age of a U238/Pb206 sample.
You are absolutely 100% wrong that you need the original ratio to calculate the age. This is another example of your ignorance of science and mathematics:
In chemical kinetics the slowest step in any reaction is the rate determining step. The conversion of uranium 238 to lead 206 is a FIRST ORDER PROCESS which can be represented by a first order differential equation. The reaction is dependent on only ONE component in the reaction. There is no requirement for a previous ratio or value because the reaction is LINEAR.




You and the idiots at the Creation Institute have used that excuse ad infinitum to prove that the age of the Earth or rocks or whatever cannot be known.
So now it's your turn - present your mathematical proof that the original isotopic ratio is required to determine the age of a U238/Pb206 sample.
edit on 21-12-2018 by Phantom423 because: (no reason given)
originally posted by: Phantom423
a reply to: cooperton
Notice in this graph they are assuming the initial ratio was 100%-0% Uranium-Lead. Let me ask you... when in the history of ever has there been found a 100% pure sample of any substance in nature? There hasn't. This never happens. There is never a 100% sample. Yet these tests assume the initial ratio to be 100%-0%. Your religion is based on assumptions that have no basis in empirical science
You and the idiots
Lol, chill. I know you think you're super smart and all dissenting opinions are really dumb, no need to reiterate that.
So now it's your turn - present your mathematical proof that the original isotopic ratio is required to determine the age of a U238/Pb206 sample.
Do you even science bro? Here is the equation to determine age of a sample based on isotopic ratios and half life:
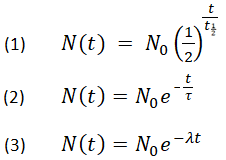
No in the equation above stands for original isotopic ratio and it is required to determine the age of a radioactive sample. I am convinced you do not know what you're talking about, nor do the two people who gave stars to your ignorant hate-filled chauvinistic rant.
Therefore the dilemma remains: scientists cannot know the original isotopic ratios, so they are left to make assumptions. The assumption that the initial ratio could have been 100%-0% is absolutely unfounded in natural science.
edit on 22-12-2018 by cooperton because: (no reason
given)
a reply to: cooperton
And you are wrong once again: N sub 0 is the MASS, not the isotopic ratio
ln N sub t/N sub 0 = -kt
where ln is the natural log of
N sub t is MASS of radioactive material at the time interval (t) divided by:
N sub 0 is the MASS of the original material
-kt
k = decay constant
t = time interval (t1/2 for half life)
And you have ZERO evidence that radioactive decay was statistically different at t minus any time you care to choose.
You really are better at dinosaurs than physics and math.
And you are wrong once again: N sub 0 is the MASS, not the isotopic ratio
ln N sub t/N sub 0 = -kt
where ln is the natural log of
N sub t is MASS of radioactive material at the time interval (t) divided by:
N sub 0 is the MASS of the original material
-kt
k = decay constant
t = time interval (t1/2 for half life)
And you have ZERO evidence that radioactive decay was statistically different at t minus any time you care to choose.
You really are better at dinosaurs than physics and math.
edit on 22-12-2018 by Phantom423 because: (no reason given)
edit on 22-12-2018 by Phantom423 because: (no reason
given)
edit on 22-12-2018 by Phantom423 because: (no reason given)
a reply to: cooperton
The graph makes no such assumption. The graph depicts a first order mechanism where the absolute dating of a sample can be determined. If the decay rates were different in the past, then samples TODAY would vary by MASS vs isotopic ratio. And they DON'T.
Notice in this graph they are assuming the initial ratio was 100%-0% Uranium-Lead. Let me ask you... when in the history of ever has there been found a 100% pure sample of any substance in nature? There hasn't. This never happens. There is never a 100% sample. Yet these tests assume the initial ratio to be 100%-0%. Your religion is based on assumptions that have no basis in empirical science
The graph makes no such assumption. The graph depicts a first order mechanism where the absolute dating of a sample can be determined. If the decay rates were different in the past, then samples TODAY would vary by MASS vs isotopic ratio. And they DON'T.
a reply to: cooperton
And I say once again: you and the idiots at the Creation Institute have bastardized science to the point where it's unrecognizable in the real world. A dissenting opinion requires evidence. You have none.
Lol, chill. I know you think you're super smart and all dissenting opinions are really dumb, no need to reiterate that.
And I say once again: you and the idiots at the Creation Institute have bastardized science to the point where it's unrecognizable in the real world. A dissenting opinion requires evidence. You have none.
originally posted by: Phantom423
a reply to: cooperton
You are absolutely 100% wrong that you need the original ratio to calculate the age.
I'm going to harp on this point, because you need to admit you are wrong. You giving a semantic merry-go-round is not going to get you out of this one. You need the original ratio of uranium-lead so you can distinguish between lead created through uranium radioactivity, and lead that was originally present in the sample:
"The simplified application of the decay equation presented above, bases age determinations on measurement of the ratio of parent : daughter isotopes. The fundamental assumption in this simplified approach is that there existed no daughter atoms at the time the radiometric clock started. This assumption is in many cases not valid, as daughter atoms certainly existed in the mineral or rock at the time the radiometric clock started. "
University of Oregon
If you can't admit you are wrong then it proves that you have lost your objectivity.
new topics
-
Thousands Of Young Ukrainian Men Trying To Flee The Country To Avoid Conscription And The War
Other Current Events: 26 minutes ago -
12 jurors selected in Trump criminal trial
US Political Madness: 3 hours ago -
Iran launches Retalliation Strike 4.18.24
World War Three: 3 hours ago -
Israeli Missile Strikes in Iran, Explosions in Syria + Iraq
World War Three: 3 hours ago -
George Knapp AMA on DI
Area 51 and other Facilities: 9 hours ago -
Not Aliens but a Nazi Occult Inspired and then Science Rendered Design.
Aliens and UFOs: 9 hours ago -
Louisiana Lawmakers Seek to Limit Public Access to Government Records
Political Issues: 11 hours ago
top topics
-
BREAKING: O’Keefe Media Uncovers who is really running the White House
US Political Madness: 16 hours ago, 25 flags -
George Knapp AMA on DI
Area 51 and other Facilities: 9 hours ago, 24 flags -
Biden--My Uncle Was Eaten By Cannibals
US Political Madness: 17 hours ago, 19 flags -
Israeli Missile Strikes in Iran, Explosions in Syria + Iraq
World War Three: 3 hours ago, 13 flags -
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest: 17 hours ago, 7 flags -
Louisiana Lawmakers Seek to Limit Public Access to Government Records
Political Issues: 11 hours ago, 7 flags -
So I saw about 30 UFOs in formation last night.
Aliens and UFOs: 15 hours ago, 6 flags -
Not Aliens but a Nazi Occult Inspired and then Science Rendered Design.
Aliens and UFOs: 9 hours ago, 5 flags -
Iran launches Retalliation Strike 4.18.24
World War Three: 3 hours ago, 5 flags -
Do we live in a simulation similar to The Matrix 1999?
ATS Skunk Works: 16 hours ago, 4 flags
active topics
-
Not Aliens but a Nazi Occult Inspired and then Science Rendered Design.
Aliens and UFOs • 12 • : BeyondKnowledge3 -
Elites disapearing
Political Conspiracies • 31 • : Degradation33 -
Israeli Missile Strikes in Iran, Explosions in Syria + Iraq
World War Three • 51 • : TheMisguidedAngel -
MULTIPLE SKYMASTER MESSAGES GOING OUT
World War Three • 47 • : SchrodingersRat -
12 jurors selected in Trump criminal trial
US Political Madness • 21 • : VictorVonDoom -
British TV Presenter Refuses To Use Guest's Preferred Pronouns
Education and Media • 64 • : Degradation33 -
Iran launches Retalliation Strike 4.18.24
World War Three • 14 • : Cloudbuster1 -
Thousands Of Young Ukrainian Men Trying To Flee The Country To Avoid Conscription And The War
Other Current Events • 0 • : Consvoli -
African "Newcomers" Tell NYC They Don't Like the Free Food or Shelter They've Been Given
Social Issues and Civil Unrest • 17 • : SchrodingersRat -
Canadian Forces bow out and loose interest in UFO’s
Aliens and UFOs • 20 • : Ophiuchus1