It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
12
share:
Have this "Lost At Sea" episode of "Man, Woman, Wild" playing.
Dude pointed out that it's possible mix about 10-20% seawater to freshwater to extend the water supply while in a survival situation. Said that the "extra electrolytes" could be of benefit too.
Chemically speaking, "electrolyte" is the proper term (for any chemicals that increase water conductivity).
Biochemically speaking, just salt in water isn't what we'd call Gatorade / Powerade "electrolytes". After all, it can be said that salt can cause dehydration, which for some adds confusion to why would athletes drink saltwater?
The answer is in how the potassium iodide / sodium chloride chemically reacts with sugars in water. I've mixed sugar and salt many times, without any sweetener, and an odd thing happens when you hit the ratio, as you cant really taste either.
For instance, you can make your own 'gatorade' electrolye drink by mixing salt and sugar together into water. Either type of iodide can work, although potassium iodide (sold as 'sodium free salt' / 'popcorn salt') as part of the formula would make for a better sports drink.
With everyday table salt + white sugar the mix ratio always seems to me that it's roughly the same amount of grains. The sugar grains are much larger, but you can pretty much eye it out by grain count and hit the right ratio. You'll know when you hit the right blend when you can no longer taste either.
I'm writing this improptu while playing this show right now, and haven't mixed up any electrolyte in a couple years, while I'm in a rush right now, so just how far adding sugar into seawater would go in a long tern survival situation. I'll have to think some more about it, and didn't find anything about adding sugar to seawater just now in the Google, but figured I'd drop this real quick and get some of you thinking about it too. I doubt that my books on marine biology and water chemistry would offer a lot of insights, as the sugar dynamic but if you can safely add "10-20%" seawater to fresh water in a survival situation then surely having a stash of clean sugar in your sea survival kit would make for an even better situation...
everydayroots.com...
Apologies for any sloppiness above, I haven't been thinking much about chemistry the past year, but there ought to be something to this. I'll dig into some sports drink papers tonight maybe. Where to look is the maximum sodium in sports drinks versus seawater composition, and in just what is happening between the sugar and iodide 'salts' in the reaction, and in the stomach.
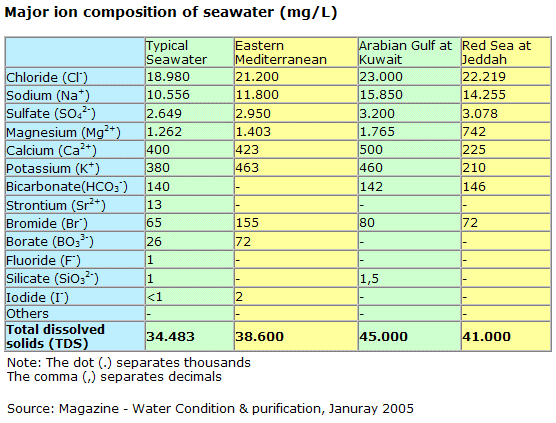
[The concentration of salt in seawater (salinity) is about 35 parts per thousand. Stated in another way, about 3.5 percent of the weight of seawater comes from the dissolved salts;] oceanservice.noaa.gov...
[A diagram summarizing the recent state of the art for exercise of durations up to 24 hours can be found in Rehrer (2001). She opted for 20 mM (1.2 g/L or 0.12% w/v) NaCl and 60 g/L (6% w/v) carbohydrate at least partly in the form of glucose polymers for an expected consumption rate of 1.5 L/h in exercise lasting 2 hours, through to twice as much NaCl and half as much carbohydrate for half the expected rate of fluid intake in exercise lasting 24 hours.] www.sportsci.org...
[When you drink salty water, from the sea or the ocean, you’re putting both salt and water into your system. The problem is that the amount of water you take in is nowhere near the amount you’ll need to expel the salt, and if you keep drinking the seawater, you’ll keep adding to the amount of salt you need to get out of your body. When you drink water from the sea or ocean, your body needs more water to get rid of the salt than it’s getting out of the seawater itself. Essentially, when you drink seawater, you’re dehydrating yourself.] morethanjustsurviving.com...
[Seawater contains salt. When humans drink seawater, their cells are thus taking in water and salt. While humans can safely ingest small amounts of salt, the salt content in seawater is much higher than what can be processed by the human body. Additionally, when we consume salt as part of our daily diets, we also drink liquids, which help to dilute the salt and keep it at a healthy level. Living cells do depend on sodium chloride (salt) to maintain the body’s chemical balances and reactions; however, too much sodium can be deadly.
Human kidneys can only make urine that is less salty than salt water. Therefore, to get rid of all the excess salt taken in by drinking seawater, you have to urinate more water than you drank. Eventually, you die of dehydration even as you become thirstier.] oceanservice.noaa.gov...
[Supplemental sodium can be crucial for elite athletes, but Rehrer and Kavouras agree that it’s not necessary unless you plan to exercise for a significant amount of time. “If your goal is just to go to the gym and do a 45-minute workout,” Kavouras says, “water is more than enough to keep you hydrated.” According to Rehrer, most people take in enough sodium by following a typical Western diet.
Finding the right amount of sugar is another tricky feat: Too much lowers the rate at which the drink empties from the stomach and enters the bloodstream, but too little means less energy and a possibly unpalatable drink given the added salt. As a result, an average sports drink contains about 3–7% sugary carbohydrate. Formulators experiment with mixing various types of sugars, such as glucose, fructose, sucrose, and maltodextrins, so as not to overload any one type of transporter in the gut.] cen.acs.org...
Dude pointed out that it's possible mix about 10-20% seawater to freshwater to extend the water supply while in a survival situation. Said that the "extra electrolytes" could be of benefit too.
Chemically speaking, "electrolyte" is the proper term (for any chemicals that increase water conductivity).
Biochemically speaking, just salt in water isn't what we'd call Gatorade / Powerade "electrolytes". After all, it can be said that salt can cause dehydration, which for some adds confusion to why would athletes drink saltwater?
The answer is in how the potassium iodide / sodium chloride chemically reacts with sugars in water. I've mixed sugar and salt many times, without any sweetener, and an odd thing happens when you hit the ratio, as you cant really taste either.
For instance, you can make your own 'gatorade' electrolye drink by mixing salt and sugar together into water. Either type of iodide can work, although potassium iodide (sold as 'sodium free salt' / 'popcorn salt') as part of the formula would make for a better sports drink.
With everyday table salt + white sugar the mix ratio always seems to me that it's roughly the same amount of grains. The sugar grains are much larger, but you can pretty much eye it out by grain count and hit the right ratio. You'll know when you hit the right blend when you can no longer taste either.
I'm writing this improptu while playing this show right now, and haven't mixed up any electrolyte in a couple years, while I'm in a rush right now, so just how far adding sugar into seawater would go in a long tern survival situation. I'll have to think some more about it, and didn't find anything about adding sugar to seawater just now in the Google, but figured I'd drop this real quick and get some of you thinking about it too. I doubt that my books on marine biology and water chemistry would offer a lot of insights, as the sugar dynamic but if you can safely add "10-20%" seawater to fresh water in a survival situation then surely having a stash of clean sugar in your sea survival kit would make for an even better situation...
everydayroots.com...
Apologies for any sloppiness above, I haven't been thinking much about chemistry the past year, but there ought to be something to this. I'll dig into some sports drink papers tonight maybe. Where to look is the maximum sodium in sports drinks versus seawater composition, and in just what is happening between the sugar and iodide 'salts' in the reaction, and in the stomach.


[The concentration of salt in seawater (salinity) is about 35 parts per thousand. Stated in another way, about 3.5 percent of the weight of seawater comes from the dissolved salts;] oceanservice.noaa.gov...
[A diagram summarizing the recent state of the art for exercise of durations up to 24 hours can be found in Rehrer (2001). She opted for 20 mM (1.2 g/L or 0.12% w/v) NaCl and 60 g/L (6% w/v) carbohydrate at least partly in the form of glucose polymers for an expected consumption rate of 1.5 L/h in exercise lasting 2 hours, through to twice as much NaCl and half as much carbohydrate for half the expected rate of fluid intake in exercise lasting 24 hours.] www.sportsci.org...
[When you drink salty water, from the sea or the ocean, you’re putting both salt and water into your system. The problem is that the amount of water you take in is nowhere near the amount you’ll need to expel the salt, and if you keep drinking the seawater, you’ll keep adding to the amount of salt you need to get out of your body. When you drink water from the sea or ocean, your body needs more water to get rid of the salt than it’s getting out of the seawater itself. Essentially, when you drink seawater, you’re dehydrating yourself.] morethanjustsurviving.com...
[Seawater contains salt. When humans drink seawater, their cells are thus taking in water and salt. While humans can safely ingest small amounts of salt, the salt content in seawater is much higher than what can be processed by the human body. Additionally, when we consume salt as part of our daily diets, we also drink liquids, which help to dilute the salt and keep it at a healthy level. Living cells do depend on sodium chloride (salt) to maintain the body’s chemical balances and reactions; however, too much sodium can be deadly.
Human kidneys can only make urine that is less salty than salt water. Therefore, to get rid of all the excess salt taken in by drinking seawater, you have to urinate more water than you drank. Eventually, you die of dehydration even as you become thirstier.] oceanservice.noaa.gov...
[Supplemental sodium can be crucial for elite athletes, but Rehrer and Kavouras agree that it’s not necessary unless you plan to exercise for a significant amount of time. “If your goal is just to go to the gym and do a 45-minute workout,” Kavouras says, “water is more than enough to keep you hydrated.” According to Rehrer, most people take in enough sodium by following a typical Western diet.
Finding the right amount of sugar is another tricky feat: Too much lowers the rate at which the drink empties from the stomach and enters the bloodstream, but too little means less energy and a possibly unpalatable drink given the added salt. As a result, an average sports drink contains about 3–7% sugary carbohydrate. Formulators experiment with mixing various types of sugars, such as glucose, fructose, sucrose, and maltodextrins, so as not to overload any one type of transporter in the gut.] cen.acs.org...
edit on 15-3-2017 by IgnoranceIsntBlisss because: (no reason given)
IgnoranceIsntBlisss
I was just talking about this with a friend the other day. I'm doing a bit of a run next week and he said to take salt water with me to replenish my electrolytes. I was just going to do that until I read your thread. Now I want to try out the video method.
Anyhow, was just wanting to say thanks for the information. I would much rather drink something like this than Gatorade.
Thanks,
blend57
I was just talking about this with a friend the other day. I'm doing a bit of a run next week and he said to take salt water with me to replenish my electrolytes. I was just going to do that until I read your thread. Now I want to try out the video method.
Anyhow, was just wanting to say thanks for the information. I would much rather drink something like this than Gatorade.
Thanks,
blend57
a reply to: blend57
If you're not using RO water, or rather if you're using chlorinated water, sprinkle in a tiny bit of Ascorbic Acid (Vitamin C). It neutralizes chlorine.
As a survivalist tip, keep it and chlorine on hand to purify water. The minimal amount of chlorine it takes to safely neutralize water, its a serious amount. Like fade you cloths serious. The chlorine will dissipate out as a gas, but that takes time. The citrus eliminates the wait.
The same scenario above with using first aid kit iodine as well. Strong taste. And again the citrus neutralizes it.
If I figure out how it would benefit a seawater trick, aside from scurvy lol, I'll be anxious to post it in here later...
I added some scratchpad data in at the end of the OP, before the edit window cut off.
I'm now thinking how much a person is sweating would be a factor. Like in the tropics during summer there might be a bunch of wiggle room, while in the COLD there might now be any.
If you're not using RO water, or rather if you're using chlorinated water, sprinkle in a tiny bit of Ascorbic Acid (Vitamin C). It neutralizes chlorine.
As a survivalist tip, keep it and chlorine on hand to purify water. The minimal amount of chlorine it takes to safely neutralize water, its a serious amount. Like fade you cloths serious. The chlorine will dissipate out as a gas, but that takes time. The citrus eliminates the wait.
The same scenario above with using first aid kit iodine as well. Strong taste. And again the citrus neutralizes it.
If I figure out how it would benefit a seawater trick, aside from scurvy lol, I'll be anxious to post it in here later...
I added some scratchpad data in at the end of the OP, before the edit window cut off.
I'm now thinking how much a person is sweating would be a factor. Like in the tropics during summer there might be a bunch of wiggle room, while in the COLD there might now be any.
edit on 15-3-2017 by IgnoranceIsntBlisss because: (no reason given)
Makes me think of this....Matter of fact a lot of things these days make me think of this movie...
originally posted by: IgnoranceIsntBlisss
a reply to: GuidedKill
I didn't even think of that.
It's got what plants need....
a reply to: GuidedKill
Nope! It's what plants CRAVE!
Errors above: 'sodium free salt' (i.e. Morton Salt Substitute) is Potassium chloride, not Potassium iodide.
duh
And 'popcorn salt' isn't inherently sodium free, as its not like its a technical specification.
Nope! It's what plants CRAVE!
Errors above: 'sodium free salt' (i.e. Morton Salt Substitute) is Potassium chloride, not Potassium iodide.
duh
And 'popcorn salt' isn't inherently sodium free, as its not like its a technical specification.
edit on 15-3-2017 by IgnoranceIsntBlisss because: (no reason given)
new topics
-
The Tories may be wiped out after the Election - Serves them Right
Regional Politics: 55 minutes ago -
So I saw about 30 UFOs in formation last night.
Aliens and UFOs: 2 hours ago -
Do we live in a simulation similar to The Matrix 1999?
ATS Skunk Works: 3 hours ago -
BREAKING: O’Keefe Media Uncovers who is really running the White House
US Political Madness: 4 hours ago -
Biden--My Uncle Was Eaten By Cannibals
US Political Madness: 5 hours ago -
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest: 5 hours ago -
The good, the Bad and the Ugly!
Diseases and Pandemics: 7 hours ago -
Russian intelligence officer: explosions at defense factories in the USA and Wales may be sabotage
Weaponry: 9 hours ago -
African "Newcomers" Tell NYC They Don't Like the Free Food or Shelter They've Been Given
Social Issues and Civil Unrest: 10 hours ago -
Russia Flooding
Fragile Earth: 11 hours ago
top topics
-
BREAKING: O’Keefe Media Uncovers who is really running the White House
US Political Madness: 4 hours ago, 18 flags -
Biden--My Uncle Was Eaten By Cannibals
US Political Madness: 5 hours ago, 15 flags -
African "Newcomers" Tell NYC They Don't Like the Free Food or Shelter They've Been Given
Social Issues and Civil Unrest: 10 hours ago, 12 flags -
Pro Hamas protesters at Columbia claim hit with chemical spray
World War Three: 16 hours ago, 11 flags -
Two Serious Crimes Committed by President JOE BIDEN that are Easy to Impeach Him For.
US Political Madness: 13 hours ago, 7 flags -
911 emergency lines are DOWN across multiple states
Breaking Alternative News: 13 hours ago, 7 flags -
Russia Flooding
Fragile Earth: 11 hours ago, 5 flags -
Former NYT Reporter Attacks Scientists For Misleading Him Over COVID Lab-Leak Theory
Education and Media: 15 hours ago, 5 flags -
So I saw about 30 UFOs in formation last night.
Aliens and UFOs: 2 hours ago, 4 flags -
"We're All Hamas" Heard at Columbia University Protests
Social Issues and Civil Unrest: 5 hours ago, 4 flags
active topics
-
The Tories may be wiped out after the Election - Serves them Right
Regional Politics • 8 • : gortex -
It has begun... Iran begins attack on Israel, launches tons of drones towards the country
World War Three • 878 • : WeMustCare -
So I saw about 30 UFOs in formation last night.
Aliens and UFOs • 8 • : BrotherKinsMan -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 523 • : Thoughtful3 -
Pro Hamas protesters at Columbia claim hit with chemical spray
World War Three • 15 • : Athetos -
Two Serious Crimes Committed by President JOE BIDEN that are Easy to Impeach Him For.
US Political Madness • 14 • : xuenchen -
Biden--My Uncle Was Eaten By Cannibals
US Political Madness • 29 • : WeMustCare -
The Fight for Election Integrity Continues -- Audits, Criminal Investigations, Legislative Reform
2024 Elections • 4135 • : matafuchs -
Elites disapearing
Political Conspiracies • 25 • : Scratchpost -
Do we live in a simulation similar to The Matrix 1999?
ATS Skunk Works • 10 • : Ravenwatcher
12
