It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
a reply to: liejunkie01
If everything remains the same, yes.
Is the ice supoosed to be there forever?
If everything remains the same, forever.
Also if the ice has been there for about ten thousand years, then how long is it supposed to last?
Actually, we are still in an ice age. The last "glacial period" ended about 10,000 years ago. Up until fairly recently, things hadn't changed much since then.
It seems we are coming out of the last ice age, starting at approximately 11,0000 years ago.
edit on 5/24/2015 by Phage because: (no reason given)
originally posted by: Phage
originally posted by: anonentity
a reply to: lostbook
Its an ice shelf, which means its resting on water, therefore displacing all the water it can, whether in the frozen state or a liquid state. It cant contribute to sea level rises . It can only dilute the salt content of the sea water.
Sort of, in a limited scope of view.
An ice shelf is a maritime extension of a glacier and as such, plays a role in slowing a glacier's advance. The collapse of an ice shelf can accelerate the movement of a glacier into the ocean. The result being an increase in water displacement.
nsidc.org...
Since Antarctica is the driest place on Earth, their would be minimal precipitation, to feed the Glaciers. So at some point wouldn't they stall.
a reply to: anonentity
When gravity does, I guess.
Or ice shelves stay in place.
So at some point wouldn't they stall.
When gravity does, I guess.
Or ice shelves stay in place.
edit on 5/24/2015 by Phage because: (no reason given)
originally posted by: Greven
Once upon a time, the skeptic argument was that CO2 measurements were uncertain - and they are. That is a pretty valid argument. However, some skeptics realized that CO2 was being dated after temperature rises. A new argument was realized - that CO2 rises because of increased temperatures - based on ice core data. Suddenly, the old valid argument hinders the new argument.
See the problem? Also, you might notice in that image you linked that the CO2 concentrations on the chart are well below current levels. So here's a question for you: if CO2 precedes temperature rise... what do you think that chart is going to show for future temperatures?
I fully understand and agree that ice core samples are not indicative of anything globally, just as my seemingly increase in harshness in winters over the past two years means nothing about the winters in Australia. But data is data, and until something is decided with verifiable certainty that these charts that show CO2 rise as a product of temperature fluctuation and not the other way around, it's at least a logical thing to consider in the overall discussion of the which-came-first part of the argument.
I'm not interested in if CO2 precedes temp rises because that's not what empirical data shows--at least not the data that my scientific brain sees as being the most correct from the best-collected data. But if that changes, I'll entertain that what-if scenario...especially if anything can convince me that 99% of climate change isn't due to a natural cycle--a cycle that is historically shown to always have anomalies as part of the upward and downward trends, and all before humanity's polluting technologies were around.
@SlapMonkey Temperature precedes CO2 in the short-term in the modern temperature record as well as the paleoclimate record. There's an interesting
graph from WoodsForTrees that you could probably Google showing CO2-changes lagging corresponding temperature-changes by a number of months. The
cause-and-effect realationship is back-to-front and yet these warmist zealots are still convinced CO2 is driving temperature; it's akin to religious
fundamentalism. Poor souls.
edit on 25-5-2015 by Nathan-D because: (no reason given)
a reply to: Nathan-D
Where's the CO2 coming from?
But you have the seasonal changes in CO2 levels (not to be confused with long term changes) reversed. Warmer temperatures increase plant growth, increased plant growth causes reduced CO2 levels, not increased. On a seasonal basis, CO2 levels are lower when it is warmer.
www.ferdinand-engelbeen.be...
Where's the CO2 coming from?
But you have the seasonal changes in CO2 levels (not to be confused with long term changes) reversed. Warmer temperatures increase plant growth, increased plant growth causes reduced CO2 levels, not increased. On a seasonal basis, CO2 levels are lower when it is warmer.
www.ferdinand-engelbeen.be...
edit on 5/25/2015 by Phage because: (no reason given)
originally posted by: Phage
a reply to: Nathan-D
Where's the CO2 coming from?
But you have the seasonal changes in CO2 levels (not to be confused with long term changes) reversed. Warmer temperatures increase plant growth, increased plant growth causes reduced CO2 levels, not increased. On a seasonal basis, CO2 levels are lower when it is warmer.
www.ferdinand-engelbeen.be...
We do have increased plant growth. I've been taking note of this in my yard.
But the CO2 is still lagging (lagging sea-surface temperature by around 11-12 months) and the cause-and-effect relationship is still backwards, not just on shorter time-scales but also longer time-scales.
originally posted by: Phage
a reply to: Nathan-D
Where's the CO2 coming from?
But you have the seasonal changes in CO2 levels (not to be confused with long term changes) reversed. Warmer temperatures increase plant growth, increased plant growth causes reduced CO2 levels, not increased. On a seasonal basis, CO2 levels are lower when it is warmer.
www.ferdinand-engelbeen.be...
In regard to your question, I would argue that the increase in CO2 is probaly natural and I'm in the middle of creating a rather in-depth post on this subject. I disagree with Ferdinand Engelbeen and I think his method of calculating an adjustment time is flawed. Engelbeen has calculated an adjustment time of over 50 years on the assumption that humans have caused the increase in atmospheric CO2 and have created an equilibrium displacement of 110ppmv and he then uses that to prove that anthropogenic CO2 is driving the atmospheric increase. But that is circular logic. The idea is also based on the assumption that natural CO2 emissions were in balance with natural sinks in the pre-industrial times and have remained so ever since. But that is unrealistic and we have no reason to believe that is the case. Engelbeen argues that the system must be out of equilibrium by 110ppmv because the observed oceanic temperature increase cannot account for the increase in atmospheric CO2 due to temperature-induced solubility changes. But that is overly simplistic because the rates of absorption and emission depend on the displacement of the atmospheric/oceanic interface from equilibrium in terms of relative pressures and temperatures. If the concentration of CO2 in the oceans increased for example this would force more CO2 into the atmosphere upon equilibrium. 'Equilibrium' is a very big word in this context. It encompasses the totality of the earth's physcial and biological properties and changes continually in accordance with a host of envrionmental parameters and beond the purely chemical relationship is affected by things such biota. For instance a complete depletion in biological activity in the ocean could be enough to increase atmospheric CO2 by 500% (Jaworowski et al 1992). Of course that will never happen, but it does suggest that small changes in ocean biology could be enough to explain the assumed 110ppmv increase since pre-industrial times.
Also Engelbeen doesn't even calculate the reduction in CO2's solubility with Henry's law (as he claims) but instead with the ice-core data.
I might use that paragraph above in my blog-post, I explained it pretty well.
edit on 30-5-2015 by Nathan-D because: (no reason given)
originally posted by: Nathan-D
@SlapMonkey Temperature precedes CO2 in the short-term in the modern temperature record as well as the paleoclimate record. There's an interesting graph from WoodsForTrees that you could probably Google showing CO2-changes lagging corresponding temperature-changes by a number of months. The cause-and-effect realationship is back-to-front and yet these warmist zealots are still convinced CO2 is driving temperature; it's akin to religious fundamentalism.
No no no no no! It's physical experiment directly supported by extensive observational evidence, not religious faith.
And again, where is the CO2 coming from, if it's from rising temperatures?
And finally ,what happens to fossil fuels being dug and burnt? That amount can be quantified.
edit on 30-5-2015 by mbkennel because: (no
reason given)
a reply to: Nathan-D
Except experimental fact shows that carbon in the oceans is increasing, not decreasing.
Today, oceans are sinks of CO2, not sources.
And yes, it's not at equilibrium now because of the rapid increase in CO2 in the atmosphere from new emissions, i.e. fossil fuel burning. There is an effective gradient causing carbon to go into the ocean, therefore acidifying it.
Except experimental fact shows that carbon in the oceans is increasing, not decreasing.
Today, oceans are sinks of CO2, not sources.
And yes, it's not at equilibrium now because of the rapid increase in CO2 in the atmosphere from new emissions, i.e. fossil fuel burning. There is an effective gradient causing carbon to go into the ocean, therefore acidifying it.
edit on 30-5-2015 by mbkennel because: (no reason
given)
I would agree that the oceans are net-sources. However the oceans can cause an increase in CO2 due to temperature-changes while at the same time absorbing more excess CO2 than they outgas from the temperature-change. To understand why this is possible you must undersatand Henry's law. If you Google "Joanne Nova Gavin Cawley" you will see an explanation I give as to why this is possible. However an increase in ocean CO2 I hope you understand is not a unique signature of CO2 dissolution and can come about naturally (though I agree our emissions are causing increased ocean CO2) from things like costal upwelling (Evans et al 2011) which can increase the CO2 concentration in the surface-ocean to 250% of atmospheric PCO2 as well the biological effects I mentioned above in my previous post. And I agree, the system is not in equilibrium, never said it was. Equilibrium (if it ever occurs; perfect equilibrium would never occur) would take many, many years. I am not suggesting that this would be a fast. Engelbeen estimates an adjustment time of around 50 years, while I think a more reasonable adjustment time is 30 years or less, for reasons I will explain in my blog-post when I finish it. Given a 30 year adjustment time, it still allows a very large portion of the atmospheric CO2 increase to be anthropogenic.
originally posted by: mbkennel
a reply to: Nathan-D
Except experimental fact shows that carbon in the oceans is increasing, not decreasing.
Today, oceans are sinks of CO2, not sources.
And yes, it's not at equilibrium now because of the rapid increase in CO2 in the atmosphere from new emissions, i.e. fossil fuel burning. There is an effective gradient causing carbon to go into the ocean, therefore acidifying it.
edit on 30-5-2015 by Nathan-D because: (no reason
given)
a reply to: Nathan-D
Seriously? It just happens to lag by one year? Why do you say that?
But the CO2 is still lagging (lagging sea-surface temperature by around 11-12 months)
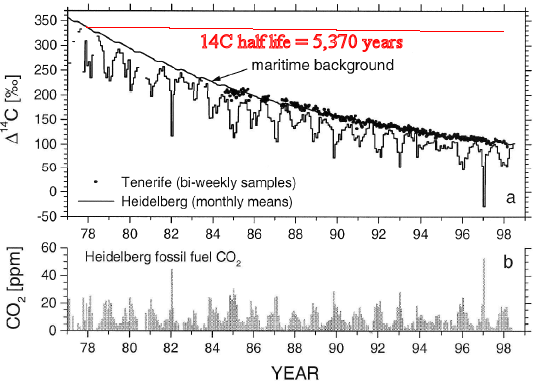
In order to claim that you would have to explain why the ratio of 12C to 13C in the atmosphere has been increasing. This increase indicates that the origin of the CO2 is plant material. You would then have to explain why the ratio of 14C to 12C has been decreasing. This decrease indicates that the origin of the CO2 is old plant material, very old. Plant material which has been buried deep underground for millions upon millions of year.
In regard to your question, I would argue that the increase in CO2 is probaly natural and I'm in the middle of creating a rather in-depth post on this subject.
What? If CO2 concentrations in the ocean are increasing it means that the oceans are absorbing more CO2 from the atmosphere than they are releasing. Pretty simple. If the oceans were releasing more CO2 than they are absorbing CO2 concentrations in the ocean would be declining. They aren't.
However the oceans can cause an increase in CO2 due to temperature-changes while at the same time absorbing more excess CO2 than they outgas from the temperature-change.
edit on 5/30/2015 by Phage because: (no reason given)
This is what we would expect if anthropogenic CO2 were accumulating in the atmosphere, these ratios would change (of course none of them are unique signatures of anthropogenic CO2 though). This argument is an argument of residence time, not adjustment time. Residence time is how long an atmospheric CO2 molecule stays in the atmosphere before it is absorbed by a sink where it is mixed indiscriminately with other CO2 and swapped places with. The isotopic ratio is not an argument of adjustment time, and it's why we can't use anthropogenic additions of nuclear-C14 (which are uneqviocally taken out of the atmosphere very fast) to prove that anthropogenic CO2 is not contributing to the atmospheric increase. The isotopic changes of atmospheric CO2 are a complete red-herring in the debate.
originally posted by: Phage
a reply to: Nathan-D
Seriously? It just happens to lag by one year? Why do you say that?
But the CO2 is still lagging (lagging sea-surface temperature by around 11-12 months)
In order to claim that you would have to explain why the ratio of 12C to 13C has been increasing. This increase indicates that the origin of the CO2 is plant material. You would then have to explain why the ratio of 14C to 12C has been decreasing. This decrease indicates that the origin of the CO2 is old plant material, very old.
In regard to your question, I would argue that the increase in CO2 is probaly natural and I'm in the middle of creating a rather in-depth post on this subject.
edit on 30-5-2015 by Nathan-D because: (no reason given)
Depending on who you ask human emissions since 1850 have amounted to about 1200Gts (or 155ppmv) while the increase in CO2 has been 120ppmv. So the oceans could theoretically still increase in concentartion while outgassing the 120ppmv. But anyway, like I said, increased CO2 concentration in the ocean is not brought about only by increased CO2 dissolution from the atmosphere, there are other factors that need to be accounted for.
originally posted by: Phage
a reply to: Nathan-D
edit on 30-5-2015 by Nathan-D because: (no reason given)
a reply to: Nathan-D
And what about those isotopic signatures?
www.abovetopsecret.com...
Oceans are not the only sink. Some of the sinks are faster than others but the trouble is, all of the sinks are becoming saturated. The more CO2 there is, the less efficient the sinks become.
So the oceans could theoretically still increase in concentartion while outgassing the 120ppmv.
What other factors? You mean there is a relatively new source of oceanic CO2 which is causing concentrations in the oceans (and secondarily the atmosphere) to increase?
But anyway, like I said, increased CO2 concentration in the ocean is not brought about only by increased CO2 dissolution from the atmosphere, there are other factors that need to be accounted for.
And what about those isotopic signatures?
www.abovetopsecret.com...
edit on 5/30/2015 by Phage because: (no reason given)
Not necessarily. The sinks are finite structures themselves so of course they must have a limited, but that limited hasn't been reached yet. The airborne fraction (i.e. the fraction of anthropogenoc CO2 assumed to have been retained in the atmosphere) has been decreasing, which means the sink rate has been increasing. Increasing by a factor of 300-400% (Hansen 2012).
originally posted by: Phage
a reply to: Nathan-D
Oceans are not the only sink. Some of the sinks are faster than others but the trouble is, all of the sinks are becoming saturated. The more CO2 there is, the less efficient the sinks become.
So the oceans could theoretically still increase in concentartion while outgassing the 120ppmv.
I am not suggesting that is in fact what is happening, but increasing the concentration of CO2 in the oceans would of course increase the atmospheric concentration because they exist in equilibrium. If you increase the concentration in the ocean you force an increase in the atmosphere, in accordance with Henry's law. Again, I am not suggesting this is happening, but the ocean concentraion can still increase and outgas CO2 as long as the amount outgassed is less than what is absorbed. The total amount of human emissions since the industrial revolution is in the order of 1,200-1,500Gts (150ppmv-190ppmv) and the total increase in atmospheric CO2 has been 120ppmv. So we should expect CO2 in the oceans to be increasing, even if the ocean was contributing signficantly to the atmospheric rise.
What other factors? You mean there is a relatively new source of oceanic CO2 which is causing concentrations in the oceans (and secondarily the atmosphere) to increase?
edit on 30-5-2015 by Nathan-D because: (no reason given)
a reply to: Nathan-D
The ratio of 12C to 13C is increasing while the ratio of 14C to 12C is decreasing. That means that the concentration of anthropogenic CO2 is increasing. You have not addressed this.
No it doesn't. It means that there has been an excess sink capacity.
The airborne fraction (i.e. the fraction of anthropogenoc CO2 assumed to have been retained in the atmosphere) has been decreasing, which means the sink rate has been increasing.
The ratio of 12C to 13C is increasing while the ratio of 14C to 12C is decreasing. That means that the concentration of anthropogenic CO2 is increasing. You have not addressed this.
Yes. And if you increase the concentration of atmospheric CO2 you increase the concentration of oceanic CO2.
If you increase the concentration in the ocean you force an increase in the atmosphere, in accordance with Henry's law.
Not without a source other than dissolved CO2.
So we should expect CO2 in the oceans to be increasing, even if they were contributing signficantly to the atmospheric increase.
edit on 5/30/2015 by Phage because: (no reason given)
Phage, ferrgoonessake. The sink rate is increasing and they are absorbing an increasingly greater fraction of anthropogenic CO2 every year. That is beyond doubt. Therefore your claim that "the more CO2 there is the less efficient the sinks become" has been proven wrong on that simple observation. The reason as to why the relative concentartion of C13 could still decrease even though the sinks are increasing is because the annual human CO2 emissions have probably increased by a factor greater than 300-400%. Also, a decrease in the relative amount of C13 in the atmosphere is not a unqiue signature of anthropogenic CO2. Other sources can leave the same signature, such as biomass decay of increased bacteriogenic methane activity.
No it doesn't. It means that there has been an excess sink capacity.
Measuring the removal rate of atmospheric isotopes doesn't tell us anything about adjustment time Phage, only residence time. Get it?
The ratio of 12C to 13C is increasing while the ratio of 14C to 12C is decreasing
Exactly, well done. I've never denined this. In fact I've specifically made clear throughout this discussion that this is what must be happening.
Yes. And if you increase the concentration of atmospheric CO2 you increase the concentration of oceanic CO2.
Did you read or comprehend anything I just wrote?
Not without some other source of CO2.
edit on 30-5-2015 by Nathan-D because:
(no reason given)
edit on 30-5-2015 by Nathan-D because: (no reason given)
a reply to: Nathan-D
www.abovetopsecret.com...
If the oceans are warming it means they can absorb less CO2, right? Doesn't that mean that the sink capacity of the oceans is decreasing?
The sink rate is increasing and they are absorbing an increasingly greater fraction of anthropogenic CO2 every year.
Yes. Exactly. And the decrease in the 14C/12C ratio shows that the source is very, very old organic material. Where do you think 14C depleted organic material comes from?
Also, a decrease in the relative amount of C13 in the atmosphere is not a unqiue signature of anthropogenic CO2. Other sources can leave the same signature, such as biomass decay of increased bacteriogenic methane activity.
www.abovetopsecret.com...
Why would 14C have a shorter residence time than C12?
Measuring the removal rate of atmospheric isotopes doesn't tell us anything about adjustment time Phage, only residence time. Get it?

Yes. I'm not sure you do though. Atmospheric CO2 levels are increasing. Where is the CO2 coming from?
Not without some other source of CO2.Did you read or comprehend anything I just wrote?
edit on 5/30/2015 by Phage because: (no reason given)
I'll refer to my post on another site for this: Google "Joanne Nova Gavin Grawley" and see the comments-section.
If the oceans are warming it means they can absorb less CO2, right? Doesn't that mean that the sink capacity of the oceans is decreasing?
That might be true. I agree that anthropogenic CO2 is accumulating in the atmosphere, but the original molecules can only accumulate based on the residence time equation (i.e R = M/S). Where R is the amount of time a CO2 molecule will remain in the atmosphere on average, M is the atmospheric CO2 mass and S is the removal rate. The atmospheric mass stands at about 3,000Gts and the removal rate is 788Gts/year from IPCC. Hence an anthropogenic CO2 molecule can only stay in the atmosphere, for an average, of: 3,000/788 = 3.8 years before being absorbed by a sink. Once absorbed by a sink it is then simply exchanged with another CO2 molecule, meaning those orginal anthropogenic molecules are no longer in the atmosphere. The atmospheric isotopic measurements tell us what we already know: that anthropogenic CO2 is being put into the atmosphere and is altering the isotopic composition. But it cannot tell us how much humans have contributed to the atmospheric increase since 1850 and does not tell us anything about the adjustment time.
Exactly. And the decrease in the 14C/12C ratio shows that the source is very, very old organic material
Measurements of C14 actually suggest that it has a longer residence time than C13 and C12 (Segalstad 1998). This could be due to slight differences in mass and hence dissolution coefficents or it could be because the heat from atmospheric nuclear testing ejected a large amout of C14 into the stratosphere where it has a 5-8 year delay to the troposhere. And most of the measurements on C14 are of nuclear-C14 not cosmic-ray produced C14.
Why would 14C have a shorter residence time that C12?
Whatever Phage. It's getting late over here, I think I'll call it a night.
Yes. I'm not sure you do though.
edit on 30-5-2015 by Nathan-D because: (no reason
given)
new topics
-
Ditching physical money
History: 1 hours ago -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 2 hours ago -
Don't take advantage of people just because it seems easy it will backfire
Rant: 2 hours ago -
VirginOfGrand says hello
Introductions: 3 hours ago -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections: 3 hours ago -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media: 6 hours ago -
Geddy Lee in Conversation with Alex Lifeson - My Effin’ Life
People: 7 hours ago -
God lived as a Devil Dog.
Short Stories: 7 hours ago -
Police clash with St George’s Day protesters at central London rally
Social Issues and Civil Unrest: 9 hours ago -
TLDR post about ATS and why I love it and hope we all stay together somewhere
General Chit Chat: 10 hours ago
top topics
-
Hate makes for strange bedfellows
US Political Madness: 12 hours ago, 18 flags -
Who guards the guards
US Political Madness: 15 hours ago, 13 flags -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media: 6 hours ago, 11 flags -
Police clash with St George’s Day protesters at central London rally
Social Issues and Civil Unrest: 9 hours ago, 8 flags -
TLDR post about ATS and why I love it and hope we all stay together somewhere
General Chit Chat: 10 hours ago, 7 flags -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections: 3 hours ago, 5 flags -
Has Tesla manipulated data logs to cover up auto pilot crash?
Automotive Discussion: 16 hours ago, 3 flags -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 2 hours ago, 2 flags -
Don't take advantage of people just because it seems easy it will backfire
Rant: 2 hours ago, 2 flags -
Geddy Lee in Conversation with Alex Lifeson - My Effin’ Life
People: 7 hours ago, 2 flags
active topics
-
Hate makes for strange bedfellows
US Political Madness • 36 • : Solvedit -
Don't take advantage of people just because it seems easy it will backfire
Rant • 4 • : VirginOfGrand -
Ditching physical money
History • 10 • : annonentity -
The Superstition of Full Moons Filling Hospitals Turns Out To Be True!
Medical Issues & Conspiracies • 21 • : VirginOfGrand -
-@TH3WH17ERABB17- -Q- ---TIME TO SHOW THE WORLD--- -Part- --44--
Dissecting Disinformation • 633 • : Justoneman -
VirginOfGrand says hello
Introductions • 1 • : VirginOfGrand -
Candidate TRUMP Now Has Crazy Judge JUAN MERCHAN After Him - The Stormy Daniels Hush-Money Case.
Political Conspiracies • 740 • : matafuchs -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections • 33 • : BernnieJGato -
Gold and silver prices....woo hoo
History • 84 • : annonentity -
The Democrats Take Control the House - Look what happened while you were sleeping
US Political Madness • 108 • : Zanti Misfit
