It looks like you're using an Ad Blocker.
Please white-list or disable AboveTopSecret.com in your ad-blocking tool.
Thank you.
Some features of ATS will be disabled while you continue to use an ad-blocker.
share:
a reply to: KrzYma
You seem to be representing a hydrogen atom and those don't have dipoles, but they do chemically bond to form H2 molecules, which don't really have an electric dipole.
However, when you add an Oxygen atom, and form H20, the two hydrogen atoms are off to one side, and this does form an electric dipole, which creates the surface tension in water drops, as well as in the videos of astronauts playing with spheres of water, where the effect is significant.
So what's wrong with that thought process? You are thinking of the electron as a discrete particle with a discrete location when orbiting a nucleus. It's more accurate to think of it as a "cloud". So it's the right thought process for a water molecule, but the wrong thought process for plain old hydrogen. Here are the shapes the electron clouds can take for hydrogen:
Wave function

Note these don't have lopsided charge like your diagram seems to, and like water molecules do.
In the water molecule the electron cloud favors the oxygen atom giving it greater negative charge, leaving the hydrogen atoms relatively depleted of electron presence and therefore a net positive charge, as represented in this diagram of electric attraction between water molecules.
Hydrogen bond
So if I understand your drawing, this type of electric attraction happens between water molecules (H2O), but not between hydrogen molecules (H2).
You seem to be representing a hydrogen atom and those don't have dipoles, but they do chemically bond to form H2 molecules, which don't really have an electric dipole.
However, when you add an Oxygen atom, and form H20, the two hydrogen atoms are off to one side, and this does form an electric dipole, which creates the surface tension in water drops, as well as in the videos of astronauts playing with spheres of water, where the effect is significant.
So what's wrong with that thought process? You are thinking of the electron as a discrete particle with a discrete location when orbiting a nucleus. It's more accurate to think of it as a "cloud". So it's the right thought process for a water molecule, but the wrong thought process for plain old hydrogen. Here are the shapes the electron clouds can take for hydrogen:
Wave function

The electron probability density for the first few hydrogen atom electron orbitals shown as cross-sections.
Note these don't have lopsided charge like your diagram seems to, and like water molecules do.
In the water molecule the electron cloud favors the oxygen atom giving it greater negative charge, leaving the hydrogen atoms relatively depleted of electron presence and therefore a net positive charge, as represented in this diagram of electric attraction between water molecules.
Hydrogen bond
So if I understand your drawing, this type of electric attraction happens between water molecules (H2O), but not between hydrogen molecules (H2).
edit on 29-8-2014 by Arbitrageur because: clarification
a reply to: Arbitrageur
Thats why i wanted distance so we could get into electron could overlap which of course follows columbs law. But im betting not like he thinks.
Thats why i wanted distance so we could get into electron could overlap which of course follows columbs law. But im betting not like he thinks.
edit on 8/29/14 by dragonridr because: (no reason given)
Thanks again. What I was referring to is that, in the ref frame of the visible light, since it is moving thru space at light or near light speed will
itself have extreme time dilation which will result in this light source itself emitting x or gamma rays
Similar scenario in the ref frame of the galaxy moving thru space greater than speed of light will result in the stars in that galaxy only emitting cosmic rays.
a reply to: Arbitrageur
Similar scenario in the ref frame of the galaxy moving thru space greater than speed of light will result in the stars in that galaxy only emitting cosmic rays.
a reply to: Arbitrageur
a reply to: Nochzwei
I think you're making that up. I would ask you the source but that's never worked before since you never have one.
I already explained what would happen, and it's not that. For the light to be blue-shifted (to say X-rays), the source would have to be moving toward you, not away. If it's moving away, it will be red-shifted. If you don't have sources for what you post, please post in the appropriate forum, such as the "gray area", where no sources are required.
I think you're making that up. I would ask you the source but that's never worked before since you never have one.
I already explained what would happen, and it's not that. For the light to be blue-shifted (to say X-rays), the source would have to be moving toward you, not away. If it's moving away, it will be red-shifted. If you don't have sources for what you post, please post in the appropriate forum, such as the "gray area", where no sources are required.
Alas but the source is me. This is cutting edge science that I am talking about, so its pertinent here itself. But if this is something that ought not
to be discussed, then its kind of a no go situation.
a reply to: Arbitrageur
a reply to: Arbitrageur
a reply to: Arbitrageur
so... you are saying those two hydrogen atoms do not attract each other by electric force, but by chemical bounds ??
is "chemical bounds" some new force or maybe another virtual particle in your theory ??
yes, you are saying this !
I will not comment the wave function plot you've posted, it has nothing to do with the reality, only mathematical calculations..
You seem to be representing a hydrogen atom and those don't have dipoles, but they do chemically bond to form H2 molecules, which don't really have an electric dipole.
so... you are saying those two hydrogen atoms do not attract each other by electric force, but by chemical bounds ??
is "chemical bounds" some new force or maybe another virtual particle in your theory ??
yes, you are saying this !
So if I understand your drawing, this type of electric attraction happens between water molecules (H2O), but not between hydrogen molecules (H2).
I will not comment the wave function plot you've posted, it has nothing to do with the reality, only mathematical calculations..
edit on
30-8-2014 by KrzYma because: (no reason given)
So you don't recall your high school chemistry? They aren't chemical bounds, they are chemical bonds, in this case covalent bonds:
originally posted by: KrzYma
so... you are saying those two hydrogen atoms do not attract each other by electric force, but by chemical bounds ??
is "chemical bounds" some new force or maybe another virtual particle in your theory ??
Molecular Orbitals-H2
Here's a diagram from that link showing the separate hydrogen atoms on the left and right, while the center diagrams represent the wave functions of how they share electrons:
The symmetric combination (called a bonding orbital) is lower in energy than the basis orbitals, and the antisymmetric combination (called an antibonding orbital) is higher. Because the H2 molecule has two electrons, they can both go in the bonding orbital, making the system lower in energy (and, hence, more stable) than two free hydrogen atoms. This is called a covalent bond.
I never said it had nothing to do with electric force, I said that it doesn't have a dipole as shown in your diagram, which is an inaccurate representation. The wave function representation is more accurate.
We have pictures now to support the math:
I will not comment the wave function plot you've posted, it has nothing to do with the reality, only mathematical calculations..
www.abovetopsecret.com...
There's a reason accepted science is accepted....it's usually because of supporting evidence.
Originally posted by VitalOverdose
reply to post by Nathwa
Well it proves that the maths we have been using to simulate atoms and the theories we have come up with about the way they work are correct. It means we are on the right track to understanding how the universe works.
[atsimg]http://files.abovetopsecret.com/images/member/48da3d162815.jpg[/atsimg]
[atsimg]http://files.abovetopsecret.com/images/member/68cbe40a92ea.gif[/atsimg]
We are indeed clever little monkeys
You can discuss "cutting edge" science with no sources in the skunk works forum, that's what it's there for; extreme theories with no sources:
originally posted by: Nochzwei
Alas but the source is me. This is cutting edge science that I am talking about, so its pertinent here itself. But if this is something that ought not to be discussed, then its kind of a no go situation.
www.abovetopsecret.com...
Since your claim is exactly opposite of experimentally confirmed mainstream science, which makes it extreme, and you have no corroboration, that's exactly the place to discuss it. This isn't the right place to discuss "scientific" claims without any sources, where I put "scientific" in quotes because if you don't have any sources better than "because I said so", it's probably not scientific.
ATS Skunk Works: This forum is dedicated to the all-important highly speculative topics that may not be substantiated by many, if any facts and span the spectrum of topics discussed on ATS. Readers and users should be aware that extreme theories without corroboration are embraced in this forum.
See? Even you imply that someone else's "say so" is questionable if that's all they've got, and it's not good enough for science. We can say the same thing about your "say so", or mine. Hopefully I can back up what I say with more than "because I said so", and if you want to post in this forum you should understand it's not skunk works, so the same would be expected of you.
originally posted by: Nochzwei
But In the case of podkletnov its only his say so
edit on 30-8-2014 by Arbitrageur because: clarification
You are a funny bloke. You are learning something new here, which you should embrace. This is how science works. But anyway each to his own. By your
own admission, there have been tests in the lab showing confirming freq change with time dilation and now you are contradicting yourself and denying
yourself.
As such let me choose when to resort to skunk works, which I may never do.
a reply to: Arbitrageur
As such let me choose when to resort to skunk works, which I may never do.
a reply to: Arbitrageur
originally posted by: Nochzwei
You are a funny bloke. You are learning something new here, which you should embrace. This is how science works. But anyway each to his own. By your own admission, there have been tests in the lab showing confirming freq change with time dilation and now you are contradicting yourself and denying yourself.
As such let me choose when to resort to skunk works, which I may never do.
a reply to: Arbitrageur
You seriously need to look at this you seem very confused.
en.wikipedia.org...
Thought id add this as well we know frequency isnt effected by speed which is exactly what your arguing. Since time is measured by the time it would take a photon to reach one point to another. meaning speed would have to vary for your assumption to be right.
arxiv.org...
edit on 8/30/14 by dragonridr because: (no reason given)
a reply to: Arbitrageur
so now you start with the spieling.. was a typo dude !
here www.abovetopsecret.com...
2 electrons is lower energy ? therefore 2 masses must be less mass, right ?
upload.wikimedia.org...
does it mean the red and blue lines are responsible for attraction ????
just because someone invented negative kinetic energy doesn't mean it is right !
I call this a fringe science even if populated by MS
anyway...
Did I said the hydrogen atoms in a bond ???
I said at any distance "d"
this is an [ atom ]
[(-)( + )] electron (-) and proton ( + ), electron is on the left side of the proton in this schema
we have 2 of them separated by any distance "d"
[(-)( + )] ---d--- [(-)( + )]
are they attracting each other by electrostatic force or not ?
so now you start with the spieling.. was a typo dude !
I never said it had nothing to do with electric force,
here www.abovetopsecret.com...
this type of electric attraction happens between water molecules (H2O), but not between hydrogen molecules (H2)
Because the H2 molecule has two electrons, they can both go in the bonding orbital, making the system lower in energy (and, hence, more stable) than two free hydrogen atoms.
2 electrons is lower energy ? therefore 2 masses must be less mass, right ?
upload.wikimedia.org...
does it mean the red and blue lines are responsible for attraction ????
just because someone invented negative kinetic energy doesn't mean it is right !
I call this a fringe science even if populated by MS
anyway...
Did I said the hydrogen atoms in a bond ???
I said at any distance "d"
edit on 30-8-2014 by KrzYma because: (no reason given)
this is an [ atom ]
[(-)( + )] electron (-) and proton ( + ), electron is on the left side of the proton in this schema
we have 2 of them separated by any distance "d"
[(-)( + )] ---d--- [(-)( + )]
are they attracting each other by electrostatic force or not ?
edit on 30-8-2014 by KrzYma because: (no reason given)
Hey I was talking about time dilation and according to Arb. freq increase of light source was measured in lab tests by moving the light source up. I do not know which lab though.
originally posted by: dragonridr
originally posted by: Nochzwei
You are a funny bloke. You are learning something new here, which you should embrace. This is how science works. But anyway each to his own. By your own admission, there have been tests in the lab showing confirming freq change with time dilation and now you are contradicting yourself and denying yourself.
As such let me choose when to resort to skunk works, which I may never do.
a reply to: Arbitrageur
You seriously need to look at this you seem very confused.
en.wikipedia.org...
Thought id add this as well we know frequency isnt effected by speed which is exactly what your arguing. Since time is measured by the time it would take a photon to reach one point to another. meaning speed would have to vary for your assumption to be right.
arxiv.org...
You can try a simple expt. Stand 3 m facing a light colored wall. Mark your eye level on the wall. Now take a flashlight and shine it on the wall. Now move the flashlight 1 m above and below your marked eye level. You will see that the reflection above your eye level is slightly brighter than the one below your eye level.
edit on 30-8-2014 by Nochzwei because: (no reason given)
Saying it's not a dipole as your diagram illustrates doesn't infer lack of electric attraction, it simply means the electric attraction is the other type, which isn't a dipole:
originally posted by: KrzYma
this type of electric attraction happens between water molecules (H2O), but not between hydrogen molecules (H2)
Chemical bond
Monatomic hydrogen H isn't a dipole, nor is diatomic hydrogen H2, but water molecules H2O have an Electric dipole moment.
A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. The bond is caused by the electrostatic force of attraction between opposite charges, either between electrons and nuclei, or as the result of a dipole attraction.
In nuclear power plants, we split the nuclear bonds in heavy atoms, and the resulting by-products have less mass. However in nuclear fusion, like fusing hydrogen into helium, the separate hydrogen has more mass than the resulting helium. So there's not one rule about whether two masses have more or less energy than one mass, you have to look at the specific atomic process to determine if there's a net mass gain or loss, but the element Iron is a clue, since we think nothing heavier is produced in routine star fusion, and that heavier elements come primarily from supernovae.
2 electrons is lower energy ? therefore 2 masses must be less mass, right ?
On the other hand, we don't measure a mass loss or gain in chemical bonds, like we do in nuclear experiments, but rather a change in energy.
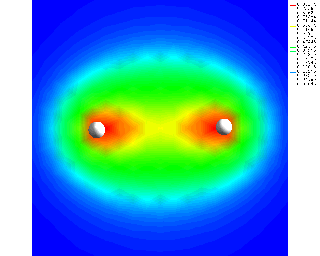
Look at the blue line in the center bottom diagram, which is a rough representation of the probability density of electron location in an H2 molecule.
does it mean the red and blue lines are responsible for attraction ????
As Eros said just because you call a dog a cat doesn't make it a cat. There is a mountain of evidence supporting the model.
just because someone invented negative kinetic energy doesn't mean it is right !
I call this a fringe science even if populated by MS
My point about no electric dipole in hydrogen applies to both monatomic hydrogen, which isn't bonded and which is what your diagram appears to inaccurately represent, and diatomic hydrogen which is when the 2 hydrogen atoms are bonded.
anyway...
Did I said the hydrogen atoms in a bond ???
I said at any distance "d"
dragonridr asked you about distance, and I didn't see where you answered his question about distance, did you? The distance does determine whether or not the two hydrogen atoms can bond, but it doesn't affect whether they are dipoles or not since neither form is a dipole.
My point was, should you choose to post extreme theories without corroboration, they would be welcome in skunk works. I don't care if you actually do that or not.
originally posted by: Nochzwei
As such let me choose when to resort to skunk works, which I may never do.
My other point is, since you don't see any such heading in this forum that extreme theories without corroboration are welcome, it's because they are not welcome here in this specific forum. So if you're going to post them at all, and I don't care if you do or don't, please post them where they are welcome, and not where they're not.
They did two experiments, one based on relative motion, which would be more relevant to your question about relative motion, and another changing the acceleration of the clock due to gravity by vertical movement. Changing the acceleration isn't quite the same as linear motion, so let's talk about linear motion.
originally posted by: Nochzwei
Hey I was talking about time dilation and according to Arb. freq increase of light source was measured in lab tests by moving the light source up. I do not know which lab though.
For two reference frames in relative linear motion, the theory and experimental results suggest that the time dilation is relative, not absolute. If you switch reference frames, it's always the other clock that seems to run slower. So we can't say which clock is actually slower, it's both and neither because it depends on reference frame.
Time dilation
When two observers are in relative uniform motion and uninfluenced by any gravitational mass, the point of view of each will be that the other's (moving) clock is ticking at a slower rate than the local clock.
Here is some information about the experiments which took place at NIST:
www.scientificamerican.com...
researchers at the National Institute of Standards and Technology (NIST) in Boulder, Colo., registered differences in the passage of time between two high-precision optical atomic clocks when one was elevated by just a third of a meter or when one was set in motion at speeds of less than 10 meters per second.
What does this have to do with anything we're discussing? Besides I tried it and I didn't notice any difference, and why should I?
You can try a simple expt. Stand 3 m facing a light colored wall. Mark your eye level on the wall. Now take a flashlight and shine it on the wall. Now move the flashlight 1 m above and below your marked eye level. You will see that the reflection above your eye level is slightly brighter than the one below your eye level.
If you anchored your feet to the ceiling and did the same thing upside-down then what would you expect to happen, and why? For me, I'd still expect to see no difference since I saw no difference right-side up.
The experiment and claimed result don't make sense to me.
edit on 30-8-2014 by Arbitrageur because: clarification
a reply to: Arbitrageur
I don't have to, I said it in my question..
you are nicely avoiding my question, just tell me what attracts the two hydrogen atoms before they go into a bond.
are you sure ?? are those lines not the representation for charges ?? those left and right animations, and the middle the interaction ??
dragonridr asked you about distance, and I didn't see where you answered his question about distance, did you? The distance does determine whether or not the two hydrogen atoms can bond, but it doesn't affect whether they are dipoles or not since neither form is a dipole.
I don't have to, I said it in my question..
you are nicely avoiding my question, just tell me what attracts the two hydrogen atoms before they go into a bond.
Look at the blue line in the center bottom diagram, which is a rough representation of the probability density of electron location in an H2 molecule.
are you sure ?? are those lines not the representation for charges ?? those left and right animations, and the middle the interaction ??
Since my last post cited the reference that the electric attraction in chemical bonds is either dipole, or between electrons and nuclei, and I said it's not dipole, then it must be the latter, right?
originally posted by: KrzYma
you are nicely avoiding my question, just tell me what attracts the two hydrogen atoms before they go into a bond.
Here is a site that explains the interaction of two H atoms to form an H2 molecule, with diagrams that are more accurate than and quite different from your drawing:
Diatomic Molecules with 1s Atomic Orbitals
Did you read the caption? It says they are electron wave function representations:
are you sure ?? are those lines not the representation for charges ?? those left and right animations, and the middle the interaction ??
en.wikipedia.org...
Of course there is a relationship between the electron wavefunction and apparent charge distribution, so I'm not sure if you're trying to make some distinction, what the point would be.
Electron wavefunctions for the 1s orbital of a lone hydrogen atom (left and right) and the corresponding bonding (bottom) and antibonding (top) molecular orbitals of the H2 molecule...
(This plot is a one-dimensional slice through the three-dimensional system.)
Here is a color representation of electron density in H2:
www.themolecularuniverse.com...
a reply to: Arbitrageur
look, I'm not asking how a bond is made, I'm asking what attracts those two!
your link says
this is not the question !
question is, why do they approach each other if not because of the electric attraction??
look, I'm not asking how a bond is made, I'm asking what attracts those two!
your link says
If two hydrogen atoms are far enough apart (> 10 Angstroms) the electron clouds are not influenced by the other atom.
If they approach each other the electrons are drawn toward the nucleus of the other atom.
An optimum distance is reached at which there is a merging or overlapping of the 1s orbitals. There is a concentration of electron probability density between the two nuclei. They will be bonded together by this sharing of electrons. A shorter distance between the nuclei would result in an increase in repulsive force between the two positive nuclei.
this is not the question !
question is, why do they approach each other if not because of the electric attraction??
They are closely related, aren't they? I'd say the site I linked to answers both questions.
originally posted by: KrzYma
a reply to: Arbitrageur
look, I'm not asking how a bond is made, I'm asking what attracts those two!
You mean if they are more than 10 Angstroms apart? They may not. If their kinetic energy is moving them apart, they won't approach each other.
question is, why do they approach each other if not because of the electric attraction??
If they get close enough because of their kinetic energy, less than 10 angstroms, then the electric charge can attract them but it's not like this:
That illustration is too crude but if you were to try to correct it, there is a greater negative charge density between the two positive charges so the negative charge wouldn't be on the left in the left atom. If you put it on the right so both negatives are in the middle it would be closer, since each is attracted to the other positive nucleus, but this is more accurate, since it's not over-simplified to that extent. It shows the negative charges are dominant between the positive charges, because the electrons are attracted to both positively charged nuclei, if they are close enough together.
originally posted by: KrzYma
we have 2 of them separated by any distance "d"
[(-)( + )] ---d--- [(-)( + )]
www.themolecularuniverse.com...
I'm not clear on what part you don't understand, so I don't know if that answers your question or not.
edit on 30-8-2014 by Arbitrageur because: clarification
originally posted by: Nochzwei
Hey I was talking about time dilation and according to Arb. freq increase of light source was measured in lab tests by moving the light source up. I do not know which lab though.
originally posted by: dragonridr
originally posted by: Nochzwei
You are a funny bloke. You are learning something new here, which you should embrace. This is how science works. But anyway each to his own. By your own admission, there have been tests in the lab showing confirming freq change with time dilation and now you are contradicting yourself and denying yourself.
As such let me choose when to resort to skunk works, which I may never do.
a reply to: Arbitrageur
You seriously need to look at this you seem very confused.
en.wikipedia.org...
Thought id add this as well we know frequency isnt effected by speed which is exactly what your arguing. Since time is measured by the time it would take a photon to reach one point to another. meaning speed would have to vary for your assumption to be right.
arxiv.org...
You can try a simple expt. Stand 3 m facing a light colored wall. Mark your eye level on the wall. Now take a flashlight and shine it on the wall. Now move the flashlight 1 m above and below your marked eye level. You will see that the reflection above your eye level is slightly brighter than the one below your eye level.
In your example it would be hard to see their wouldnt be a difference in wavelength and you would see no measurable shift However i think your trying to discuss gravity time dilation. This isnt a change in frequency its an expansion or contraction of space time. Just like in the earlier post i gave you the link.The deeper i am the the earths gravity well the more space is curved. There would be a fractional change in frequency the equation is 1/2 (v/c)^2. this is used for our correction in GPS satellites. But the frequency isnt actually changing whats happening is its being stretched or shrank depending on which direction because it actually has to travel further or shorter depending on how much space is warped.In your example moving around a flashlight isnt going to cause a frequency shift you cant move the flashlight fast enough. But theoretically if you could move your flashlight fast enough we would see a spectral shift. Do not think of it as time slowing or speeding up but the distance our photons have to travel relative to us as expanding or contracting.
a reply to: Arbitrageur
Well the key here is there is two different bonds we could discuss depending on distance the electrons are from each other. You could have a sigma bond or a pi bond what would determine this is their velocity upon contact. Not sure if we really want to discuss the differences other than to say electron cloud in sigma bond is symmetrical about the line joining the two nuclei....while electron cloud of pi bond is unsymmetrical. And a sigma bond overlapping is quite large and hence it is a strong bond however a pi bond overlapping is small and creates a weak bond.
Now outside of 10 angstroms these electrons will not bond without colliding them with sufficient velocity we need energy to create the bond. In his example gravity would eventually bring our electrons together if they had zero velocity but than we would have two hydrogen atoms sitting in deep space hanging out.
Well the key here is there is two different bonds we could discuss depending on distance the electrons are from each other. You could have a sigma bond or a pi bond what would determine this is their velocity upon contact. Not sure if we really want to discuss the differences other than to say electron cloud in sigma bond is symmetrical about the line joining the two nuclei....while electron cloud of pi bond is unsymmetrical. And a sigma bond overlapping is quite large and hence it is a strong bond however a pi bond overlapping is small and creates a weak bond.
Now outside of 10 angstroms these electrons will not bond without colliding them with sufficient velocity we need energy to create the bond. In his example gravity would eventually bring our electrons together if they had zero velocity but than we would have two hydrogen atoms sitting in deep space hanging out.
When you move the flashlight up, it is travelling a greater dist thru space also its moved into a region where gravity is a wee bit less. Both these
will cause its time ( universes own btw ) to be wee bit dilated, that is why its slightly brighter. Besides space is not curved in any way as
hypothesized by GR.
GPS receivers correction is empirically applied correction to mans chronometer time. Kind of a thumb rule.
a reply to: dragonridr
GPS receivers correction is empirically applied correction to mans chronometer time. Kind of a thumb rule.
a reply to: dragonridr
edit on 31-8-2014 by Nochzwei because: (no reason given)
new topics
-
Ditching physical money
History: 51 minutes ago -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 1 hours ago -
Don't take advantage of people just because it seems easy it will backfire
Rant: 1 hours ago -
VirginOfGrand says hello
Introductions: 2 hours ago -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections: 2 hours ago -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media: 5 hours ago -
Geddy Lee in Conversation with Alex Lifeson - My Effin’ Life
People: 6 hours ago -
God lived as a Devil Dog.
Short Stories: 6 hours ago -
Police clash with St George’s Day protesters at central London rally
Social Issues and Civil Unrest: 8 hours ago -
TLDR post about ATS and why I love it and hope we all stay together somewhere
General Chit Chat: 9 hours ago
top topics
-
Hate makes for strange bedfellows
US Political Madness: 11 hours ago, 18 flags -
Who guards the guards
US Political Madness: 14 hours ago, 13 flags -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media: 5 hours ago, 10 flags -
Police clash with St George’s Day protesters at central London rally
Social Issues and Civil Unrest: 8 hours ago, 8 flags -
TLDR post about ATS and why I love it and hope we all stay together somewhere
General Chit Chat: 9 hours ago, 7 flags -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections: 2 hours ago, 4 flags -
Has Tesla manipulated data logs to cover up auto pilot crash?
Automotive Discussion: 15 hours ago, 3 flags -
One Flame Throwing Robot Dog for Christmas Please!
Weaponry: 1 hours ago, 2 flags -
Don't take advantage of people just because it seems easy it will backfire
Rant: 1 hours ago, 2 flags -
Geddy Lee in Conversation with Alex Lifeson - My Effin’ Life
People: 6 hours ago, 2 flags
active topics
-
Terrifying Encounters With The Black Eyed Kids
Paranormal Studies • 69 • : AlongCameaSpider -
Should Biden Replace Harris With AOC On the 2024 Democrat Ticket?
2024 Elections • 32 • : YourFaceAgain -
Candidate TRUMP Now Has Crazy Judge JUAN MERCHAN After Him - The Stormy Daniels Hush-Money Case.
Political Conspiracies • 739 • : WeMustCare -
My wife just had a very powerful prophetic dream - massive war in Israel...
The Gray Area • 16 • : AlongCameaSpider -
Why did Phizer team with nanobot maker
Medical Issues & Conspiracies • 20 • : AlongCameaSpider -
University student disciplined after saying veganism is wrong and gender fluidity is stupid
Education and Media • 21 • : YourFaceAgain -
Remember These Attacks When President Trump 2.0 Retribution-Justice Commences.
2024 Elections • 46 • : WeMustCare -
Ditching physical money
History • 2 • : GENERAL EYES -
Breaking Baltimore, ship brings down bridge, mass casualties
Other Current Events • 468 • : IndieA -
Mood Music Part VI
Music • 3093 • : TheDiscoKing